Phosphorylation-Dependent Assembly and Coordination of the DNA Damage Checkpoint Apparatus by Rad4(Topbp1.).
Qu, M., Rappas, M., Wardlaw, C.P., Garcia, V., Ren, J.Y., Day, M., Carr, A.M., Oliver, A.W., Du, L.L., Pearl, L.H.(2013) Mol Cell 51: 723
- PubMed: 24074952
- DOI: https://doi.org/10.1016/j.molcel.2013.08.030
- Primary Citation of Related Structures:
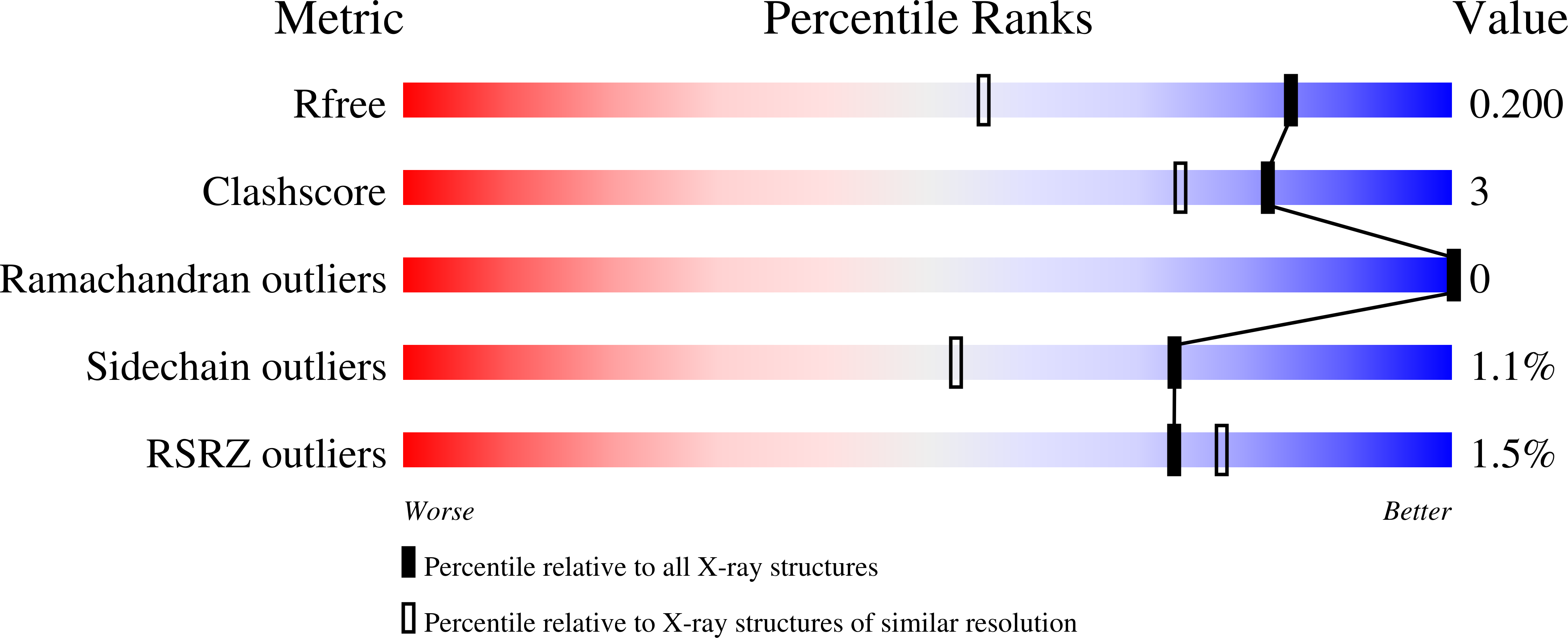
4BMC, 4BMD, 4BU0, 4BU1 - PubMed Abstract:
The BRCT-domain protein Rad4(TopBP1) facilitates activation of the DNA damage checkpoint in Schizosaccharomyces pombe by physically coupling the Rad9-Rad1-Hus1 clamp, the Rad3(ATR) -Rad26(ATRIP) kinase complex, and the Crb2(53BP1) mediator. We have now determined crystal structures of the BRCT repeats of Rad4(TopBP1), revealing a distinctive domain architecture, and characterized their phosphorylation-dependent interactions with Rad9 and Crb2(53BP1). We identify a cluster of phosphorylation sites in the N-terminal region of Crb2(53BP1) that mediate interaction with Rad4(TopBP1) and reveal a hierarchical phosphorylation mechanism in which phosphorylation of Crb2(53BP1) residues Thr215 and Thr235 promotes phosphorylation of the noncanonical Thr187 site by scaffolding cyclin-dependent kinase (CDK) recruitment. Finally, we show that the simultaneous interaction of a single Rad4(TopBP1) molecule with both Thr187 phosphorylation sites in a Crb2(53BP1) dimer is essential for establishing the DNA damage checkpoint.
Organizational Affiliation:
National Institute of Biological Sciences, 7 Science Park Road, ZGC Life Science Park, Beijing 102206, China.