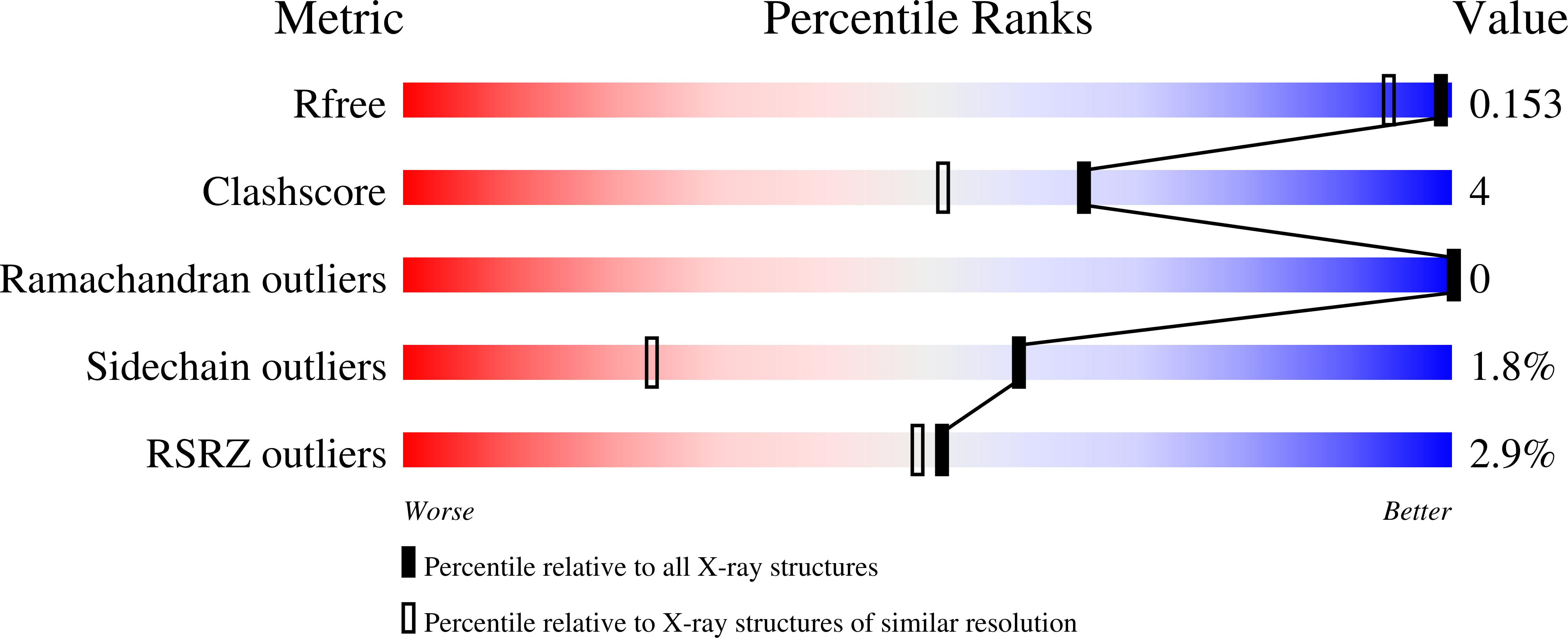
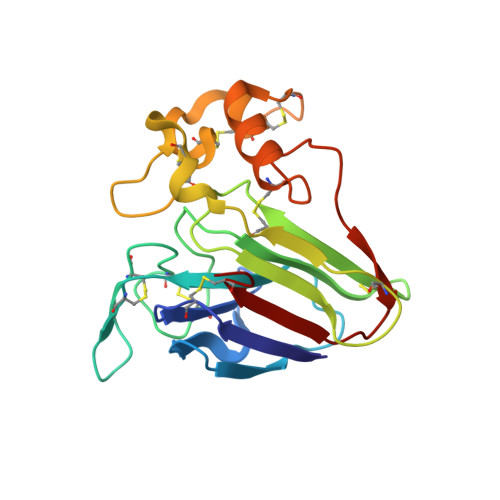
Clicked Europium Dipicolinate Complexes for Protein X-Ray Structure Determination.
Talon, R., Nauton, L., Canet, J.-L., Kahn, R., Girard, E., Gautier, A.(2012) Chem Commun (Camb) 48: 11886
- PubMed: 23123834
- DOI: https://doi.org/10.1039/c2cc36982f
- Primary Citation of Related Structures:
4BAD, 4BAF, 4BAL, 4BAP, 4BAR - PubMed Abstract:
New trisdipicolinic acid-lanthanide complexes are reported as phasing agents for X-ray crystallography of proteins. It is demonstrated that CuAAC modifications allow protein co-crystallization with low concentration of lanthanide complexes leading to an accurate structure determination.
Organizational Affiliation:
CEA, DSV, Institut de Biologie Structurale (IBS), 41 rue Jules Horowitz, Grenoble F-38027, France.