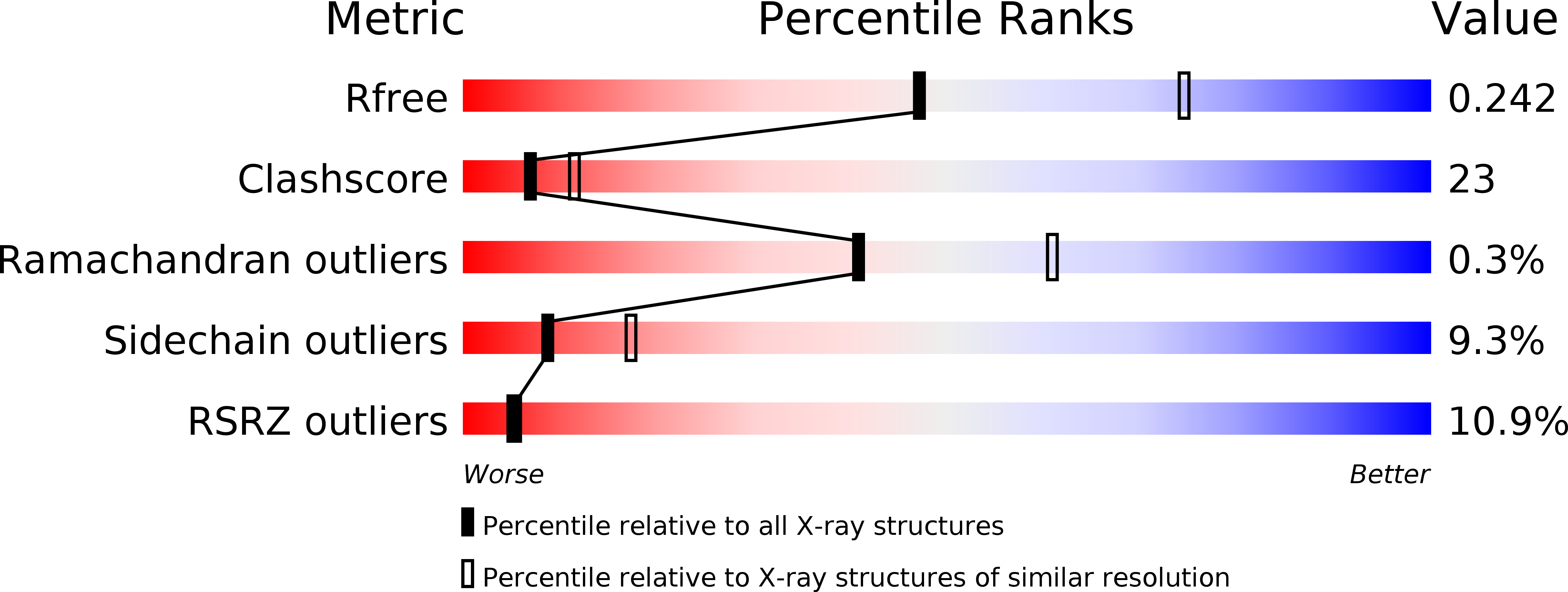
The Mitotic Kinesin Eg5 Overcomes Inhibition to the Phase I/II Clinical Candidate Sb743921 by an Allosteric Resistance Mechanism.
Talapatra, S.K., Anthony, N.G., Mackay, S.P., Kozielski, F.(2013) J Med Chem 56: 6317
- PubMed: 23875972
- DOI: https://doi.org/10.1021/jm4006274
- Primary Citation of Related Structures:
4A1Z, 4A28, 4AS7, 4B7B, 4BXN - PubMed Abstract:
Development of drug resistance during cancer chemotherapy is one of the major causes of chemotherapeutic failure for the majority of clinical agents. The aim of this study was to investigate the underlying molecular mechanism of resistance developed by the mitotic kinesin Eg5 against the potent second-generation ispinesib analogue SB743921 (1), a phase I/II clinical candidate. Biochemical and biophysical data demonstrate that point mutations in the inhibitor-binding pocket decrease the efficacy of 1 by several 1000-fold. Surprisingly, the structures of wild-type and mutant Eg5 in complex with 1 display no apparent structural changes in the binding configuration of the drug candidate. Furthermore, ITC and modeling approaches reveal that resistance to 1 is not through conventional steric effects at the binding site but through reduced flexibility and changes in energy fluctuation pathways through the protein that influence its function. This is a phenomenon we have called "resistance by allostery".
Organizational Affiliation:
The Beatson Institute for Cancer Research, Garscube Estate, Switchback Road, Bearsden, Glasgow G61 1BS, Scotland, U.K. s.talapatra@ed.ac.uk