Characterization of the Chitinolytic Machinery of Enterococcus Faecalis V583 and High Resolution Structure of its Oxidative Cbm33 Enzyme
Vaaje-Kolstad, G., Bohle, L.A., Gaseidnes, S., Dalhus, B., Bjoras, M., Mathiesen, G., Eijsink, V.G.H.(2012) J Mol Biol 416: 239
- PubMed: 22210154
- DOI: https://doi.org/10.1016/j.jmb.2011.12.033
- Primary Citation of Related Structures:
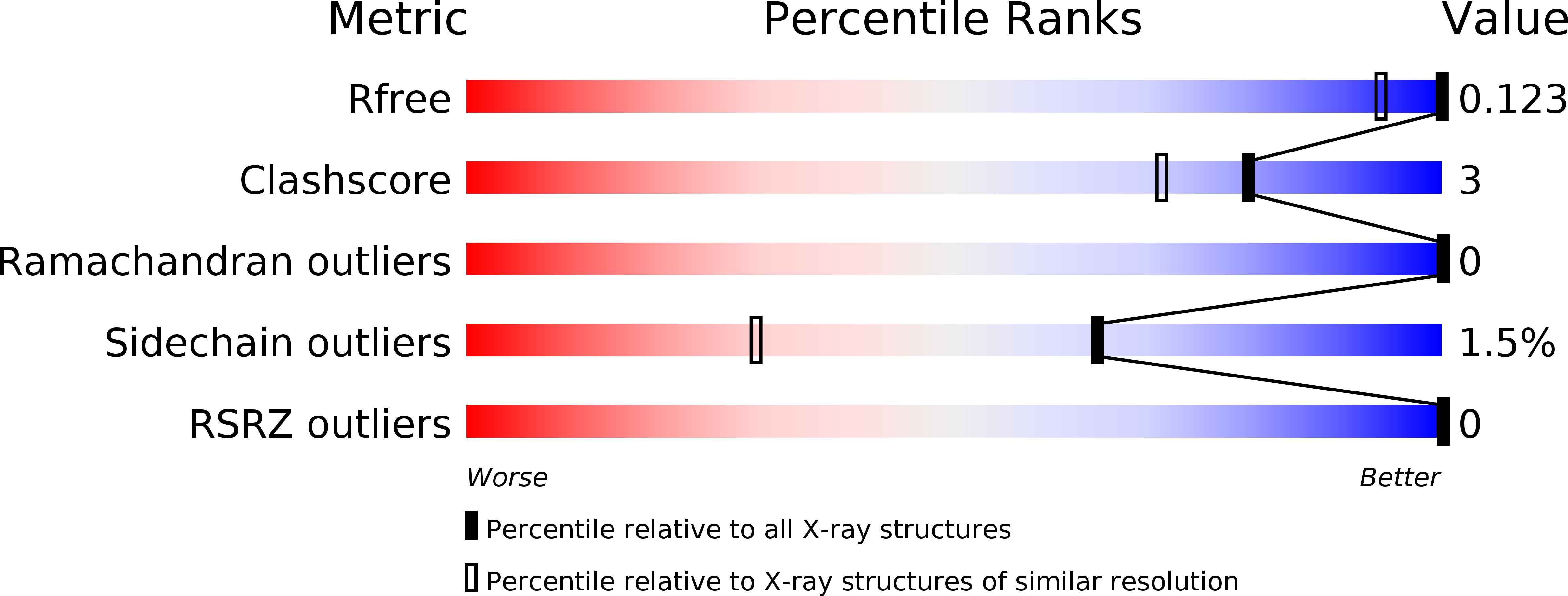
4A02 - PubMed Abstract:
Little information exists for the ability of enterococci to utilize chitin as a carbon source. We show that Enterococcus faecalis V583 can grow on chitin, and we describe two proteins, a family 18 chitinase (ef0361; EfChi18A) and a family 33 CBM (carbohydrate binding module) (ef0362; EfCBM33A) that catalyze chitin conversion in vitro. Various types of enzyme activity assays showed that EfChi18A has functional properties characteristic of an endochitinase. EfCBM33A belongs to a recently discovered family of enzymes that cleave glycosidic bonds via an oxidative mechanism and that act synergistically with classical hydrolytic enzymes (i.e., chitinases). The structure and function of this protein were probed in detail. An ultra-high-resolution crystal structure of EfCBM33A revealed details of a conserved binding surface that is optimized to interact with chitin and contains the catalytic center. Chromatography and mass spectrometry analyses of product formation showed that EfCBM33A cleaves chitin via the oxidative mechanism previously described for CBP21 from Serratia marcescens. Metal-depletion studies showed that EfCBM33A is a copper enzyme. In the presence of an external electron donor, EfCBM33A boosted the activity of EfChi18A, and combining the two enzymes led to rapid and complete conversion of β-chitin to chitobiose. This study provides insight into the structure and function of the CBM33 family of enzymes, which, together with their fungal counterpart called GH61, currently receive considerable attention in the biomass processing field.
Organizational Affiliation:
Department of Chemistry, Biotechnology and Food Science, Norwegian University of Life Sciences, N-1432 Ås, Norway. gustko@umb.no