An alternative allosteric regulation mechanism of an acidophilic l-lactate dehydrogenase from Enterococcus mundtii 15-1A.
Matoba, Y., Miyasako, M., Matsuo, K., Oda, K., Noda, M., Higashikawa, F., Kumagai, T., Sugiyama, M.(2014) FEBS Open Bio 4: 834-847
- PubMed: 25379380
- DOI: https://doi.org/10.1016/j.fob.2014.08.006
- Primary Citation of Related Structures:
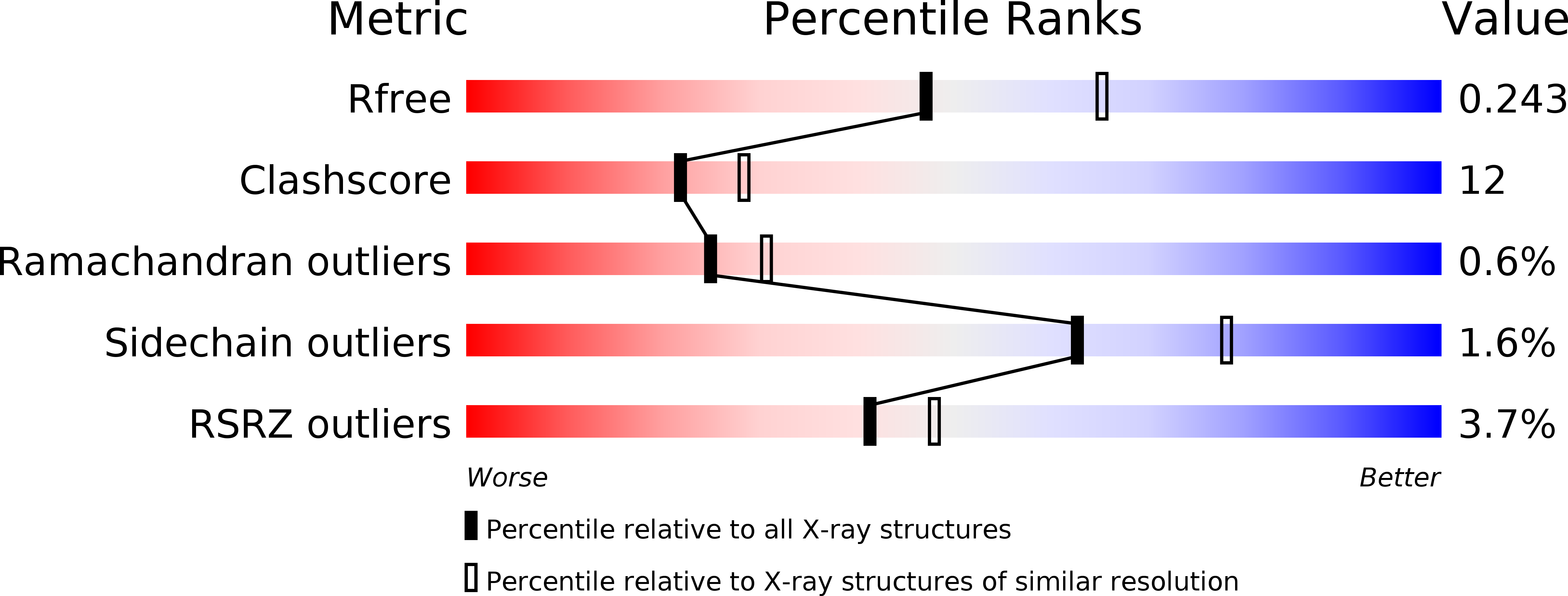
3WSV, 3WSW - PubMed Abstract:
A plant-derived Enterococcus mundtii 15-1A, that has been previously isolated from Brassica rapa L. subsp. nipposinica (L.H. Bailey) Hanelt var. linearifolia by our group, possesses two kinds of l-lactate dehydrogenase (l-LDH): LDH-1 and LDH-2. LDH-1 was activated under low concentration of fluctose-1,6-bisphosphate (FBP) at both pH 5.5 and 7.5. Although LDH-2 was also activated under the low concentration of FBP at pH 5.5, a high concentration of FBP is necessary to activate it at pH 7.5. The present study shows the crystal structures of the acidophilic LDH-2 in a complex with and without FBP and NADH. Although the tertiary structure of the ligands-bound LDH-2 is similar to that of the active form of other bacterial l-LDHs, the structure without the ligands is different from that of any other previously determined l-LDHs. Major structural alterations between the two structures of LDH-2 were observed at two regions in one subunit. At the N-terminal parts of the two regions, the ligands-bound form takes an α-helical structure, while the form without ligands displays more disordered and extended structures. A vacuum-ultraviolet circular dichroism analysis showed that the α-helix content of LDH-2 in solution is approximately 30% at pH 7.5, which is close to that in the crystal structure of the form without ligands. A D241N mutant of LDH-2, which was created by us to easily form an α-helix at one of the two parts, exhibited catalytic activity even in the absence of FBP at both pH 5.5 and 7.5.
Organizational Affiliation:
Department of Molecular Microbiology and Biotechnology, Graduate School of Biomedical & Health Sciences, Hiroshima University, Kasumi 1-2-3, Minami-ku, Hiroshima 734-8551, Japan.