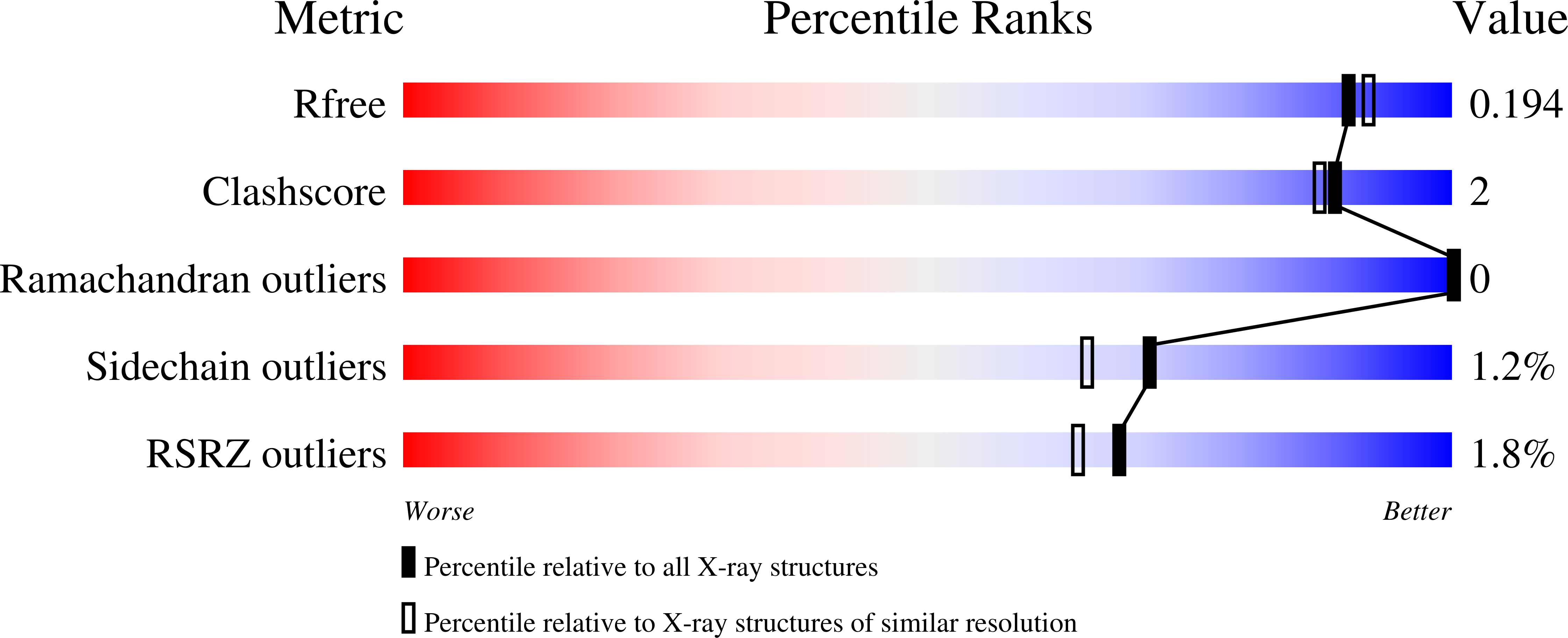
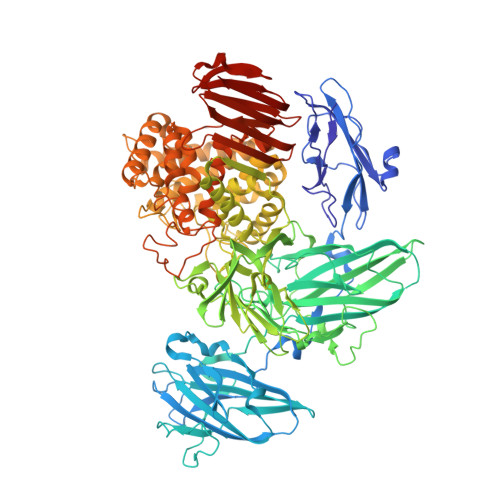
The structure of a Streptomyces avermitilis alpha-L-rhamnosidase reveals a novel carbohydrate-binding module CBM67 within the six-domain arrangement.
Fujimoto, Z., Jackson, A., Michikawa, M., Maehara, T., Momma, M., Henrissat, B.F., Gilbert, H.J., Kaneko, S.(2013) J Biol Chem 288: 12376-12385
- PubMed: 23486481
- DOI: https://doi.org/10.1074/jbc.M113.460097
- Primary Citation of Related Structures:
3W5M, 3W5N - PubMed Abstract:
α-L-rhamnosidases hydrolyze α-linked L-rhamnosides from oligosaccharides or polysaccharides. We determined the crystal structure of the glycoside hydrolase family 78 Streptomyces avermitilis α-L-rhamnosidase (SaRha78A) in its free and L-rhamnose complexed forms, which revealed the presence of six domains N, D, E, F, A, and C. In the ligand complex, L-rhamnose was bound in the proposed active site of the catalytic module, revealing the likely catalytic mechanism of SaRha78A. Glu(636) is predicted to donate protons to the glycosidic oxygen, and Glu(895) is the likely catalytic general base, activating the nucleophilic water, indicating that the enzyme operates through an inverting mechanism. Replacement of Glu(636) and Glu(895) resulted in significant loss of α-rhamnosidase activity. Domain D also bound L-rhamnose in a calcium-dependent manner, with a KD of 135 μm. Domain D is thus a non-catalytic carbohydrate binding module (designated SaCBM67). Mutagenesis and structural data identified the amino acids in SaCBM67 that target the features of L-rhamnose that distinguishes it from the other major sugars present in plant cell walls. Inactivation of SaCBM67 caused a substantial reduction in the activity of SaRha78A against the polysaccharide composite gum arabic, but not against aryl rhamnosides, indicating that SaCBM67 contributes to enzyme function against insoluble substrates.
Organizational Affiliation:
Biomolecular Research Unit, National Institute of Agrobiological Sciences, 2-1-2 Kannondai, Tsukuba 305-8602, Japan. zui@affrc.go.jp