Structural basis for cell-cycle-dependent nuclear import mediated by the karyopherin Kap121p.
Kobayashi, J., Matsuura, Y.(2013) J Mol Biol 425: 1852-1868
- PubMed: 23541588
- DOI: https://doi.org/10.1016/j.jmb.2013.02.035
- Primary Citation of Related Structures:
3W3T, 3W3U, 3W3V, 3W3W, 3W3X, 3W3Y, 3W3Z - PubMed Abstract:
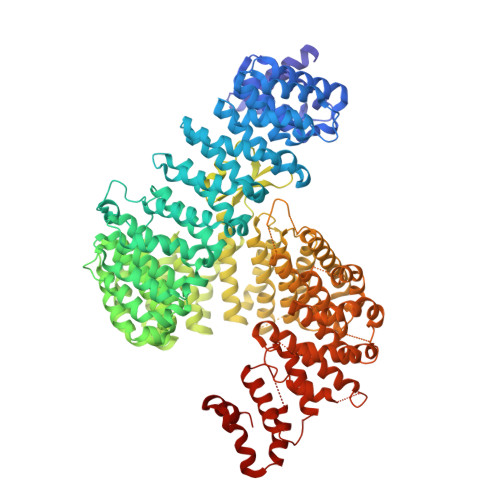
Kap121p (also known as Pse1p) is an essential karyopherin that mediates nuclear import of a plethora of cargoes including cell cycle regulators, transcription factors, and ribosomal proteins in Saccharomyces cerevisiae. It has been proposed that the spindle assembly checkpoint signaling triggers molecular rearrangements of nuclear pore complexes and thereby arrests Kap121p-mediated nuclear import at metaphase, while leaving import mediated by other karyopherins unaffected. The Kap121p-specific import inhibition is required for normal progression through mitosis. To understand the structural basis for Kap121p-mediated nuclear import and its unique regulatory mechanism during mitosis, we determined crystal structures of Kap121p in isolation and also in complex with either its import cargoes or nucleoporin Nup53p or RanGTP. Kap121p has a superhelical structure composed of 24 HEAT repeats. The structures of Kap121p-cargo complexes define a non-conventional nuclear localization signal (NLS) that has a consensus sequence of KV/IxKx1-2K/H/R. The structure of Kap121p-Nup53p complex shows that cargo and Nup53p compete for the same high-affinity binding site, explaining how Nup53p binding forces cargo release when the Kap121p-binding site of Nup53p is exposed during mitosis. Comparison of the NLS and RanGTP complexes reveals that RanGTP binding not only occludes the cargo-binding site but also forces Kap121p into a conformation that is incompatible with NLS recognition.
Organizational Affiliation:
Division of Biological Science, Graduate School of Science, Nagoya University, Japan.