Structural basis for down regulation of tight junction by PDZ-domain containing E3-Ubiquitin ligase
Akiyoshi, Y., Nakakura, Y., Hamada, D., Goda, N., Tenno, T., Narita, H., Nakagawa, A., Furuse, M., Suzuki, M., Hiroaki, H.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| E3 ubiquitin-protein ligase LNX | 94 | Mus musculus | Mutation(s): 0 Gene Names: Lnx1 | 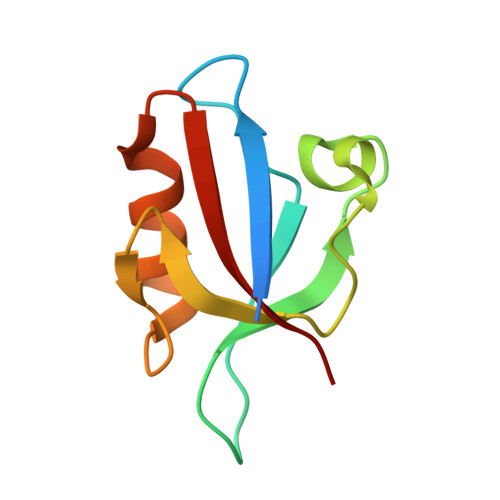 | |
UniProt | |||||
Find proteins for O70263 (Mus musculus) Explore O70263 Go to UniProtKB: O70263 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O70263 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 60.493 | α = 90 |
| b = 51.21 | β = 120 |
| c = 33.291 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| MOLREP | phasing |
| PHENIX | refinement |
| HKL-2000 | data reduction |
| SCALEPACK | data scaling |