Structural and functional analysis of the transcriptional regulator Rv3066 of Mycobacterium tuberculosis.
Bolla, J.R., Do, S.V., Long, F., Dai, L., Su, C.C., Lei, H.T., Chen, X., Gerkey, J.E., Murphy, D.C., Rajashankar, K.R., Zhang, Q., Yu, E.W.(2012) Nucleic Acids Res 40: 9340-9355
- PubMed: 22821564
- DOI: https://doi.org/10.1093/nar/gks677
- Primary Citation of Related Structures:
3V6G, 3V78 - PubMed Abstract:
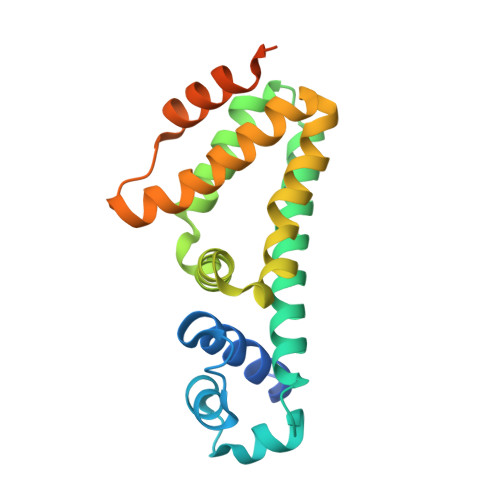
The Mmr multidrug efflux pump recognizes and actively extrudes a broad range of antimicrobial agents, and promotes the intrinsic resistance to these antimicrobials in Mycobacterium tuberculosis. The expression of Mmr is controlled by the TetR-like transcriptional regulator Rv3066, whose open reading frame is located downstream of the mmr operon. To understand the structural basis of Rv3066 regulation, we have determined the crystal structures of Rv3066, both in the absence and presence of bound ethidium, revealing an asymmetric homodimeric two-domain molecule with an entirely helical architecture. The structures underscore the flexibility and plasticity of the regulator essential for multidrug recognition. Comparison of the apo-Rv3066 and Rv3066-ethidium crystal structures suggests that the conformational changes leading to drug-mediated derepression is primarily due to a rigid body rotational motion within the dimer interface of the regulator. The Rv3066 regulator creates a multidrug-binding pocket, which contains five aromatic residues. The bound ethidium is found buried within the multidrug-binding site, where extensive aromatic stacking interactions seemingly govern the binding. In vitro studies reveal that the dimeric Rv3066 regulator binds to a 14-bp palindromic inverted repeat sequence in the nanomolar range. These findings provide new insight into the mechanisms of ligand binding and Rv3066 regulation.
Organizational Affiliation:
Department of Chemistry, College of Veterinary Medicine, Iowa State University, Ames, IA 50011, USA.