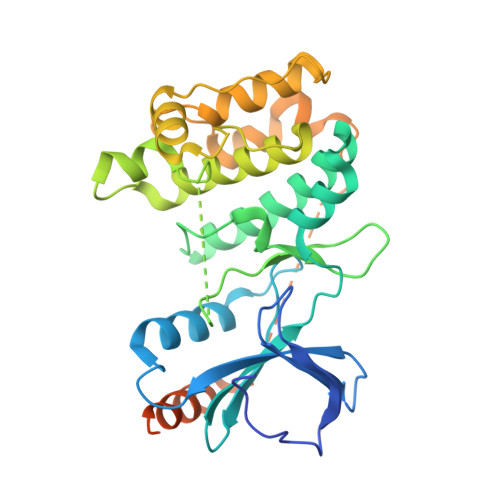
Structural basis for basal activity and autoactivation of abscisic acid (ABA) signaling SnRK2 kinases.
Ng, L.M., Soon, F.F., Zhou, X.E., West, G.M., Kovach, A., Suino-Powell, K.M., Chalmers, M.J., Li, J., Yong, E.L., Zhu, J.K., Griffin, P.R., Melcher, K., Xu, H.E.(2011) Proc Natl Acad Sci U S A 108: 21259-21264
- PubMed: 22160701
- DOI: https://doi.org/10.1073/pnas.1118651109
- Primary Citation of Related Structures:
3UC3, 3UC4 - PubMed Abstract:
Abscisic acid (ABA) is an essential hormone that controls plant growth, development, and responses to abiotic stresses. Central for ABA signaling is the ABA-mediated autoactivation of three monomeric Snf1-related kinases (SnRK2.2, -2.3, and -2.6). In the absence of ABA, SnRK2s are kept in an inactive state by forming physical complexes with type 2C protein phosphatases (PP2Cs). Upon relief of this inhibition, SnRK2 kinases can autoactivate through unknown mechanisms. Here, we report the crystal structures of full-length Arabidopsis thaliana SnRK2.3 and SnRK2.6 at 1.9- and 2.3-Å resolution, respectively. The structures, in combination with biochemical studies, reveal a two-step mechanism of intramolecular kinase activation that resembles the intermolecular activation of cyclin-dependent kinases. First, release of inhibition by PP2C allows the SnRK2s to become partially active because of an intramolecular stabilization of the catalytic domain by a conserved helix in the kinase regulatory domain. This stabilization enables SnRK2s to gain full activity by activation loop autophosphorylation. Autophosphorylation is more efficient in SnRK2.6, which has higher stability than SnRK2.3 and has well-structured activation loop phosphate acceptor sites that are positioned next to the catalytic site. Together, these data provide a structural framework that links ABA-mediated release of PP2C inhibition to activation of SnRK2 kinases.
Organizational Affiliation:
Laboratory of Structural Sciences, Van Andel Research Institute, Grand Rapids, MI 49503, USA.