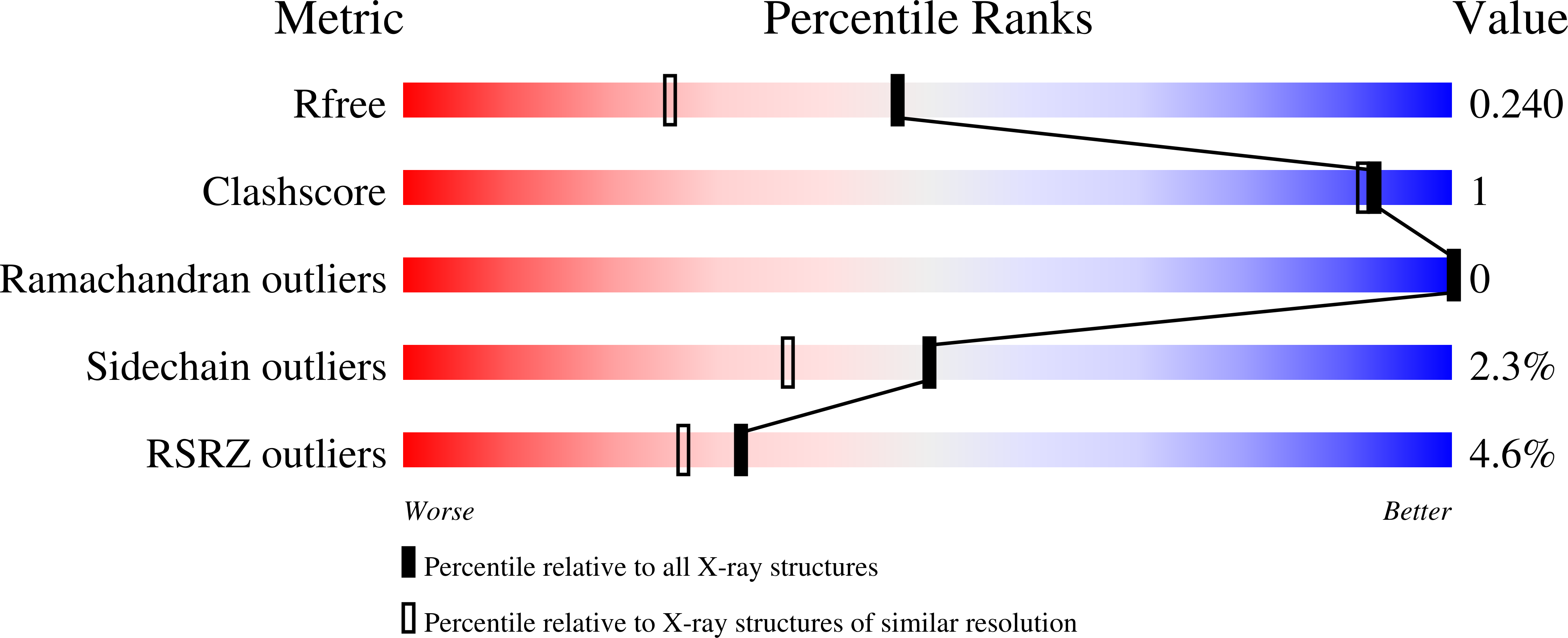
Mechanism of disruption of the Amt-GlnK complex by P(II)-mediated sensing of 2-oxoglutarate.
Maier, S., Schleberger, P., Lu, W., Wacker, T., Pfluger, T., Litz, C., Andrade, S.L.(2011) PLoS One 6: e26327-e26327
- PubMed: 22039461
- DOI: https://doi.org/10.1371/journal.pone.0026327
- Primary Citation of Related Structures:
3T9Z, 3TA0, 3TA1, 3TA2 - PubMed Abstract:
GlnK proteins regulate the active uptake of ammonium by Amt transport proteins by inserting their regulatory T-loops into the transport channels of the Amt trimer and physically blocking substrate passage. They sense the cellular nitrogen status through 2-oxoglutarate, and the energy level of the cell by binding both ATP and ADP with different affinities. The hyperthermophilic euryarchaeon Archaeoglobus fulgidus possesses three Amt proteins, each encoded in an operon with a GlnK ortholog. One of these proteins, GlnK2 was recently found to be incapable of binding 2-OG, and in order to understand the implications of this finding we conducted a detailed structural and functional analysis of a second GlnK protein from A. fulgidus, GlnK3. Contrary to Af-GlnK2 this protein was able to bind both ATP/2-OG and ADP to yield inactive and functional states, respectively. Due to the thermostable nature of the protein we could observe the exact positioning of the notoriously flexible T-loops and explain the binding behavior of GlnK proteins to their interaction partner, the Amt proteins. A thermodynamic analysis of these binding events using microcalorimetry evaluated by microstate modeling revealed significant differences in binding cooperativity compared to other characterized P(II) proteins, underlining the diversity and adaptability of this class of regulatory signaling proteins.
Organizational Affiliation:
Institut für organische Chemie und Biochemie, Albert-Ludwigs-Universität Freiburg, Freiburg, Germany.