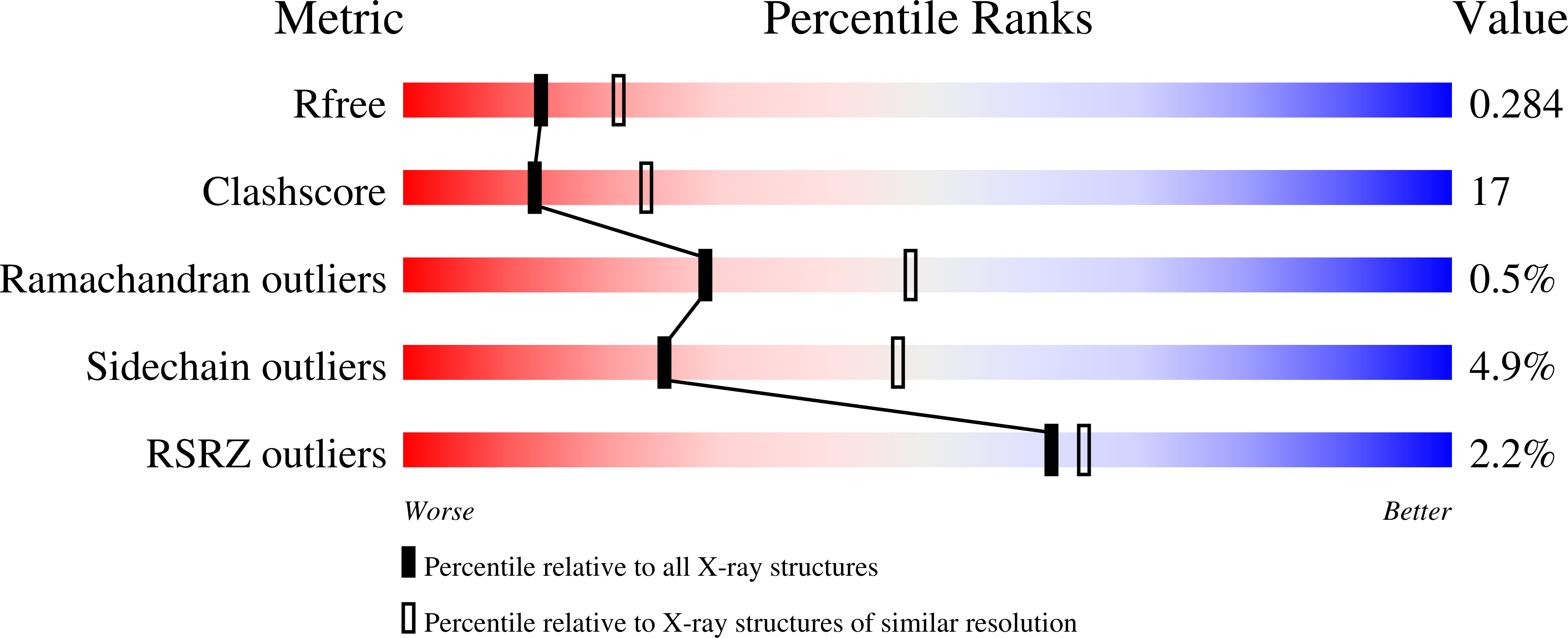
Structural characterization of BVU_3255, a methyltransferase from human intestine antibiotic resistant pathogen Bacteroides vulgatus
Kumar, V., Sivaraman, J.(2011) J Struct Biol
- PubMed: 21872662
- DOI: https://doi.org/10.1016/j.jsb.2011.08.007
- Primary Citation of Related Structures:
3T7R, 3T7S, 3T7T - PubMed Abstract:
Methylation is important for various cellular activities. To date, there is no report of any methyltransferase structure from the human intestine antibiotic resistant pathogen Bacteroides vulgatus. The protein BVU_3255 from B. vulgatus ATCC 8482 belongs to a SAM-dependent methyltransferase. Here, we report the crystal structure of apo BVU_3255, and its complexes with SAM and SAH, which revealed a typical class I Rossmann Fold Methyltransferase. Isothermal titration calorimetric studies showed that both SAM and SAH bind with equal affinity. The structural and sequence analysis suggested that BVU_3255 is a small molecule methyltransferase and involved in methylating the intermediates in ubiquinone biosynthesis pathway.
Organizational Affiliation:
Department of Biological Sciences, National University of Singapore, Singapore, Republic of Singapore.