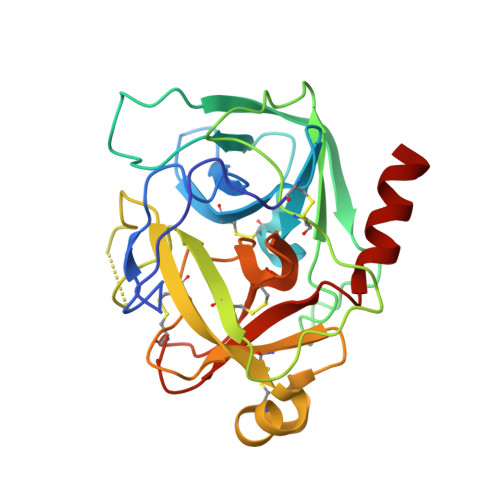
Structural insights into chymotrypsin inhibition by the Kunitz-type inhibitor-1 from the marine invertebrate Stichodactyla helianthus
Garcia-Fernandez, R., Dominguez, R., Oberthuer, D., Pons, T., Gonzalez-Gonzalez, Y., Chavez, M.A., Betzel, C., Redecke, L.To be published.