X-ray evidence of a native state with increased compactness populated by tryptophan-less B. licheniformis beta-lactamase.
Risso, V.A., Acierno, J.P., Capaldi, S., Monaco, H.L., Ermacora, M.R.(2012) Protein Sci 21: 964-976
- PubMed: 22496053
- DOI: https://doi.org/10.1002/pro.2076
- Primary Citation of Related Structures:
3SOI - PubMed Abstract:
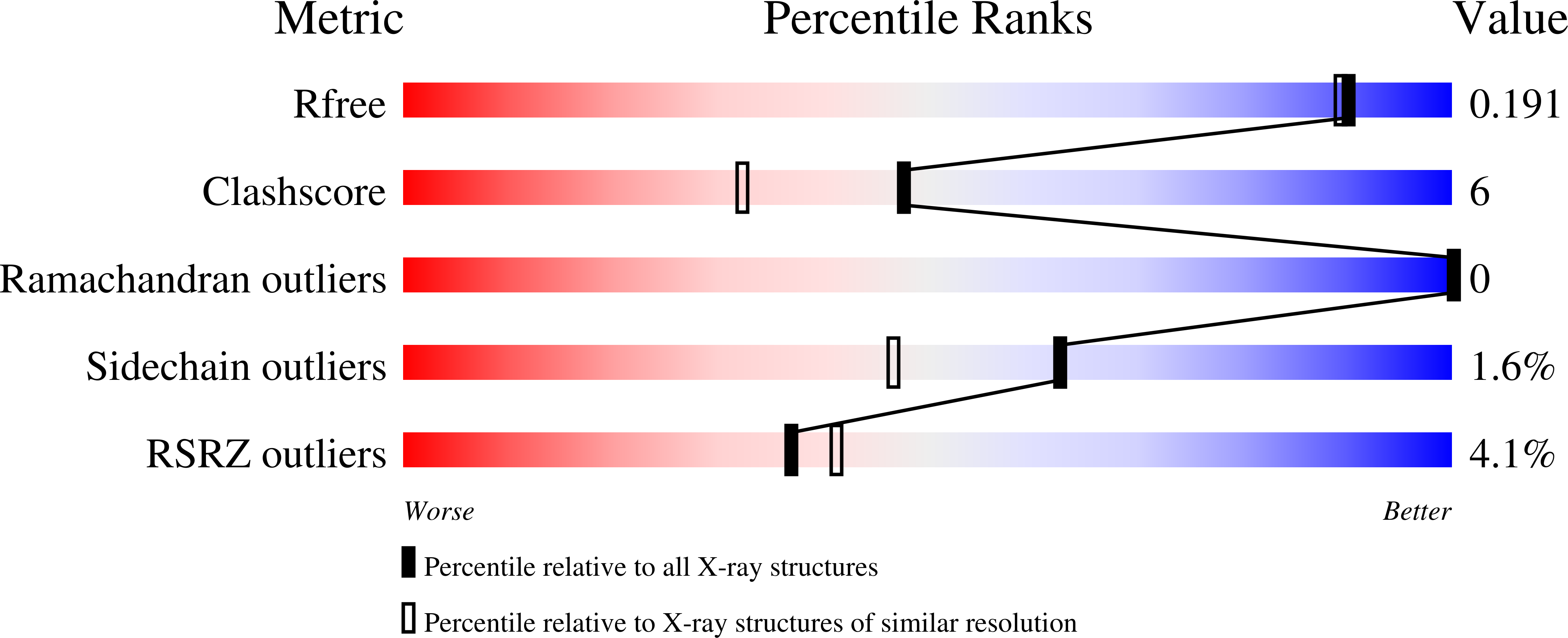
β-lactamases confer antibiotic resistance, one of the most serious world-wide health problems, and are an excellent theoretical and experimental model in the study of protein structure, dynamics and evolution. Bacillus licheniformis exo-small penicillinase (ESP) is a Class-A β-lactamase with three tryptophan residues located in the protein core. Here, we report the 1.7-Å resolution X-ray structure, catalytic parameters, and thermodynamic stability of ESP(ΔW), an engineered mutant of ESP in which phenylalanine replaces the wild-type tryptophan residues. The structure revealed no qualitative conformational changes compared with thirteen previously reported structures of B. licheniformis β-lactamases (RMSD = 0.4-1.2 Å). However, a closer scrutiny showed that the mutations result in an overall more compact structure, with most atoms shifted toward the geometric center of the molecule. Thus, ESP(ΔW) has a significantly smaller radius of gyration (R(g)) than the other B. licheniformis β-lactamases characterized so far. Indeed, ESP(ΔW) has the smallest R(g) among 126 Class-A β-lactamases in the Protein Data Bank (PDB). Other measures of compactness, like the number of atoms in fixed volumes and the number and average of noncovalent distances, confirmed the effect. ESP(ΔW) proves that the compactness of the native state can be enhanced by protein engineering and establishes a new lower limit to the compactness of the Class-A β-lactamase fold. As the condensation achieved by the native state is a paramount notion in protein folding, this result may contribute to a better understanding of how the sequence determines the conformational variability and thermodynamic stability of a given fold.
Organizational Affiliation:
Departamento de Ciencia y Tecnología, Universidad Nacional de Quilmes, Roque Sáenz Peña 325, 1876 Bernal, Buenos Aires, Argentina.