Total chemical synthesis of biologically active vascular endothelial growth factor.
Mandal, K., Kent, S.B.(2011) Angew Chem Int Ed Engl 50: 8029-8033
- PubMed: 21744452
- DOI: https://doi.org/10.1002/anie.201103237
- Primary Citation of Related Structures:
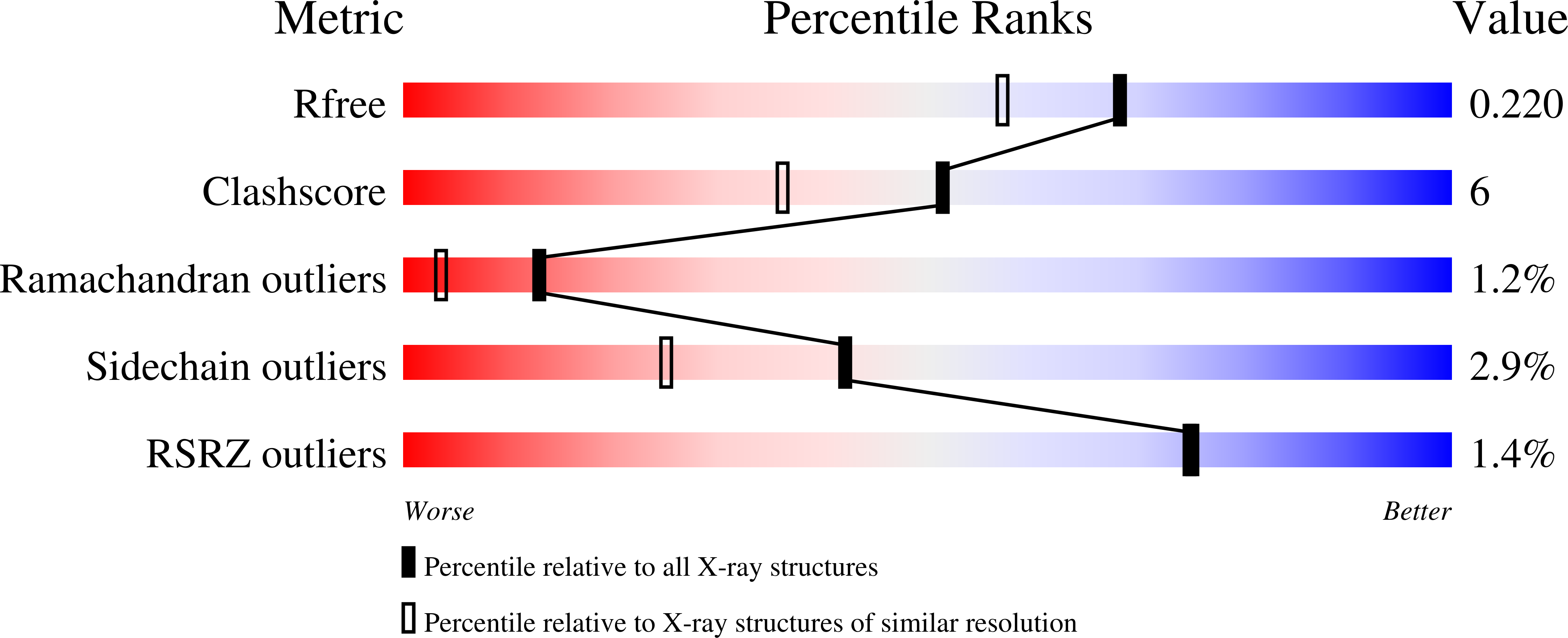
3QTK - PubMed Abstract:
Efficient access: the 204-residue covalent-dimer vascular endothelial growth factor with full mitogenic activity was prepared from three unprotected peptide segments by one-pot native chemical ligations. The covalent structure of the synthetic VEGF was confirmed by precise mass measurement, and the three-dimensional structure of the synthetic protein was determined by high-resolution X-ray crystallography.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Institute for Biophysical Dynamics, The University of Chicago, IL 60637, USA.