Redirecting cell-type specific cytokine responses with engineered interleukin-4 superkines.
Junttila, I.S., Creusot, R.J., Moraga, I., Bates, D.L., Wong, M.T., Alonso, M.N., Suhoski, M.M., Lupardus, P., Meier-Schellersheim, M., Engleman, E.G., Utz, P.J., Fathman, C.G., Paul, W.E., Garcia, K.C.(2012) Nat Chem Biol 8: 990-998
- PubMed: 23103943
- DOI: https://doi.org/10.1038/nchembio.1096
- Primary Citation of Related Structures:
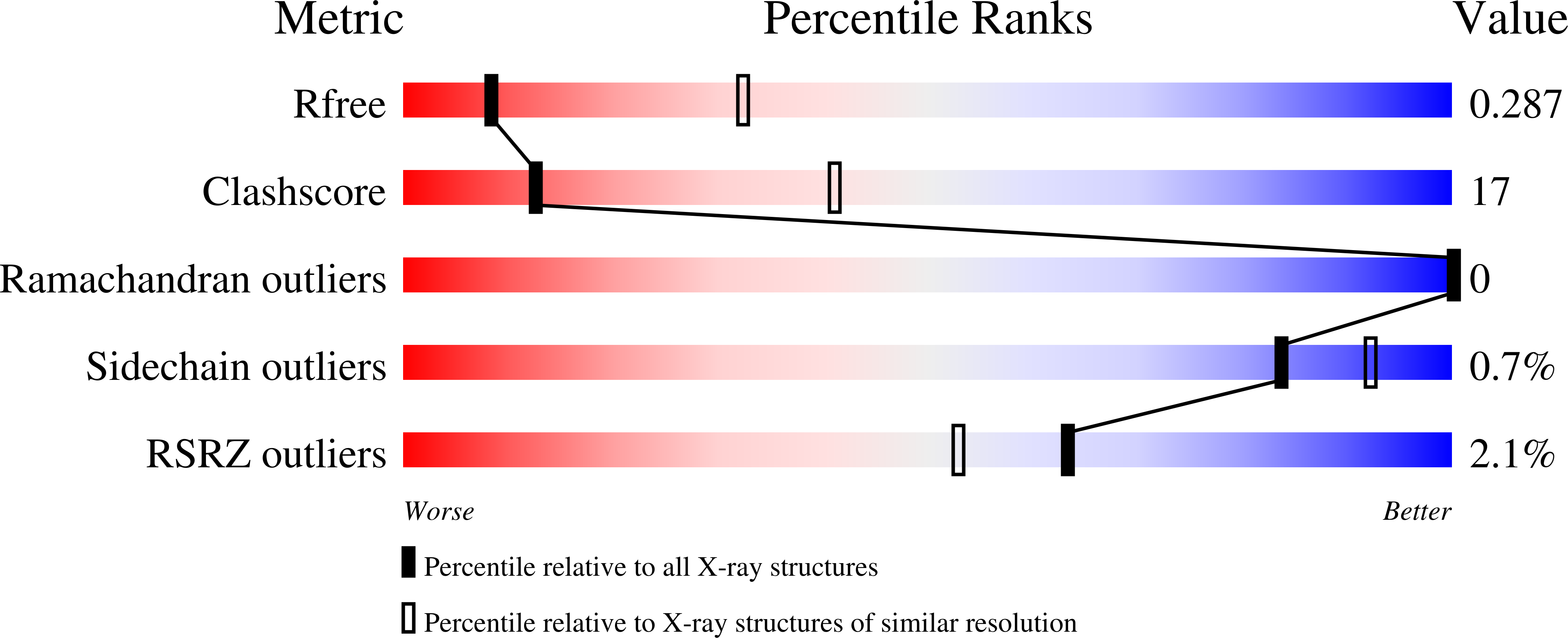
3QB7 - PubMed Abstract:
Cytokines dimerize their receptors, with the binding of the 'second chain' triggering signaling. In the interleukin (IL)-4 and IL-13 system, different cell types express varying numbers of alternative second receptor chains (γc or IL-13Rα1), forming functionally distinct type I or type II complexes. We manipulated the affinity and specificity of second chain recruitment by human IL-4. A type I receptor-selective IL-4 'superkine' with 3,700-fold higher affinity for γc was three- to ten-fold more potent than wild-type IL-4. Conversely, a variant with high affinity for IL-13Rα1 more potently activated cells expressing the type II receptor and induced differentiation of dendritic cells from monocytes, implicating the type II receptor in this process. Superkines showed signaling advantages on cells with lower second chain numbers. Comparative transcriptional analysis reveals that the superkines induce largely redundant gene expression profiles. Variable second chain numbers can be exploited to redirect cytokines toward distinct cell subsets and elicit new actions, potentially improving the selectivity of cytokine therapy.
Organizational Affiliation:
Laboratory of Immunology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.