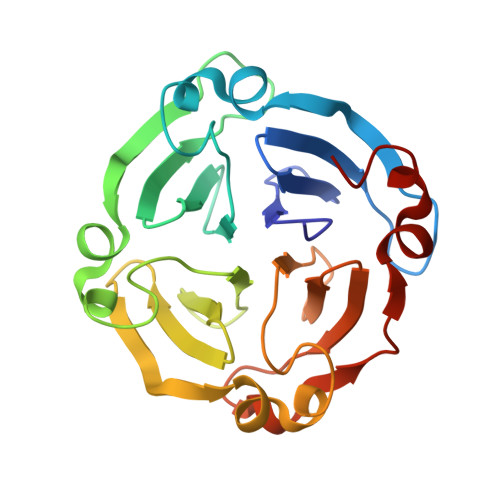
The structure of a haemopexin-fold protein from cow pea (Vigna unguiculata) suggests functional diversity of haemopexins in plants
Gaur, V., Chanana, V., Jain, A., Salunke, D.M.(2011) Acta Crystallogr Sect F Struct Biol Cryst Commun 67: 193-200
- PubMed: 21301085
- DOI: https://doi.org/10.1107/S1744309110051250
- Primary Citation of Related Structures:
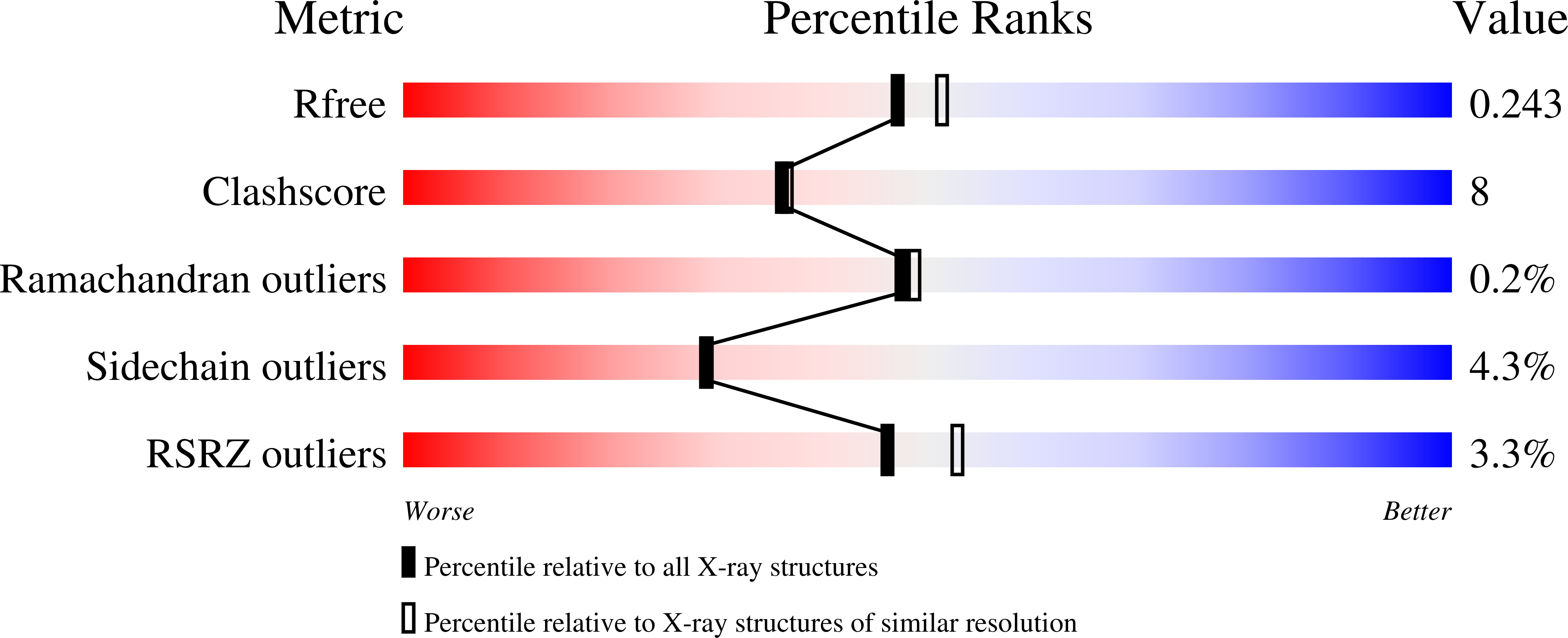
3OYO - PubMed Abstract:
The haemopexin fold is present in almost all life forms and is utilized for carrying out diverse physiological functions. The structure of CP4, a haemopexin-fold protein from cow pea (Vigna unguiculata), was determined at 2.1 Å resolution. The protein exists as a monomer both in solution and in the crystal. The structure revealed a typical four-bladed β-propeller topology. The protein exhibits 42% sequence similarity to LS-24 from Lathyrus sativus, with substantial differences in the surface-charge distribution and in the oligomeric state. A structure-based sequence analysis of haemopexin-fold proteins of plant and mammalian origin established a sequence signature associated with the haemopexin motif. This signature sequence enabled the identification of other proteins with possible haemopexin-like topology of both plant and animal origin. Although CP4 shares a structural fold with LS-24 and other haemopexins, biochemical studies indicated possible functional differences between CP4 and LS-24. While both of these proteins exhibit spermine-binding potential, CP4 does not bind to haem, unlike LS-24.
Organizational Affiliation:
National Institute of Immunology, Aruna Asaf Ali Marg, New Delhi, India.