Crystal structure of ARFGAP1-ARF1 fusion protein
Wang, H., Tong, Y., Nedyalkova, L., Tempel, W., Guan, X., Crombet, L., Arrowsmith, C.H., Edwards, A.M., Bountra, C., Weigelt, J., Bochkarev, A., Park, H.To be published.
Experimental Data Snapshot
Starting Models: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ADP-ribosylation factor GTPase-activating protein 1, ADP-ribosylation factor 1 | 329 | Homo sapiens | Mutation(s): 0 Gene Names: ARFGAP1, ARF1GAP, ARF1 | 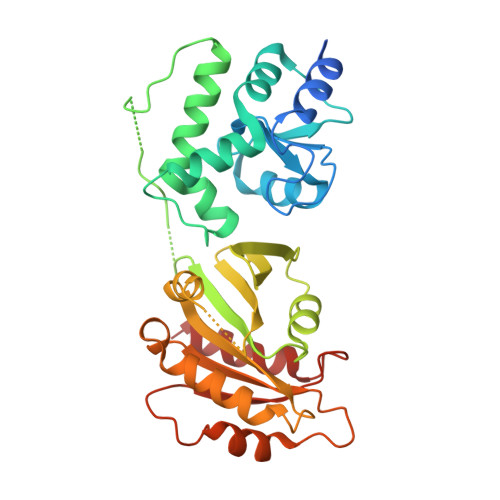 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P84077 (Homo sapiens) Explore P84077 Go to UniProtKB: P84077 | |||||
PHAROS: P84077 GTEx: ENSG00000143761 | |||||
Find proteins for Q8N6T3 (Homo sapiens) Explore Q8N6T3 Go to UniProtKB: Q8N6T3 | |||||
PHAROS: Q8N6T3 GTEx: ENSG00000101199 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | P84077Q8N6T3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GDP Query on GDP | D [auth A], F [auth B] | GUANOSINE-5'-DIPHOSPHATE C10 H15 N5 O11 P2 QGWNDRXFNXRZMB-UUOKFMHZSA-N |  | ||
| ZN Query on ZN | C [auth A], E [auth B] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 79.298 | α = 90 |
| b = 129.161 | β = 90 |
| c = 157.685 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| PHASER | phasing |
| BUSTER-TNT | refinement |
| PDB_EXTRACT | data extraction |
| BUSTER | refinement |