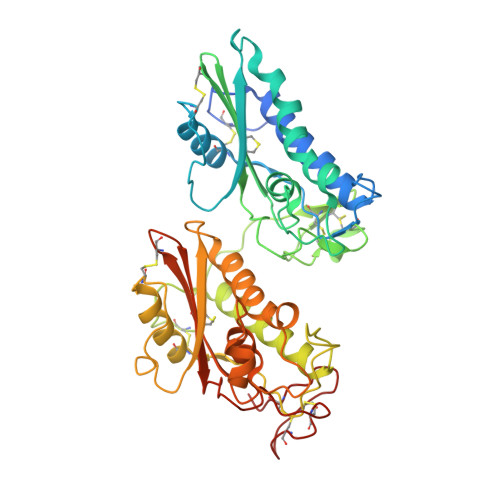
Structure of a two-CAP-domain protein from the human hookworm parasite Necator americanus.
Asojo, O.A.(2011) Acta Crystallogr D Biol Crystallogr 67: 455-462
- PubMed: 21543848
- DOI: https://doi.org/10.1107/S0907444911008560
- Primary Citation of Related Structures:
3NT8 - PubMed Abstract:
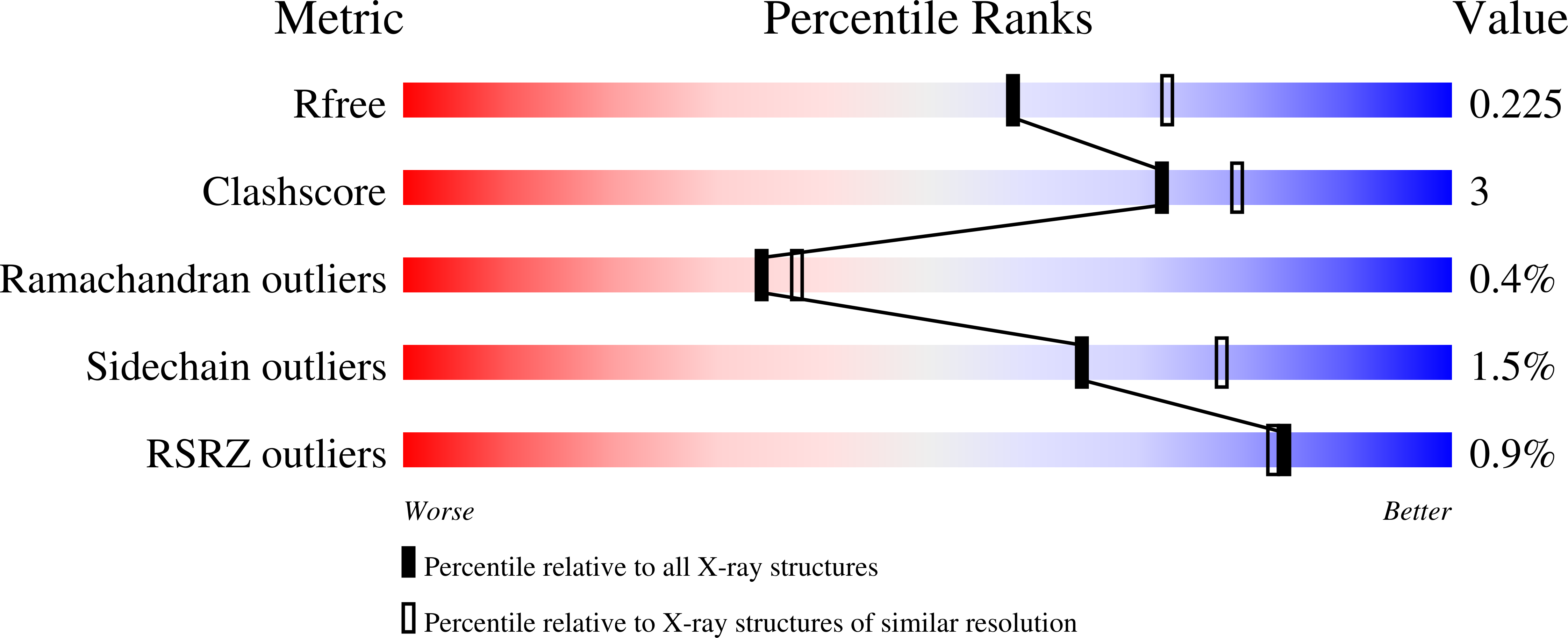
Major proteins secreted by the infective larval stage hookworms upon host entry include Ancylostoma secreted proteins (ASPs), which are characterized by one or two CAP (cysteine-rich secretory protein/antigen 5/pathogenesis related-1) domains. The CAP domain has been reported in diverse phylogenetically unrelated proteins, but has no confirmed function. The first structure of a two-CAP-domain protein, Na-ASP-1, from the major human hookworm parasite Necator americanus was refined to a resolution limit of 2.2 Å. The structure was solved by molecular replacement (MR) using Na-ASP-2, a one-CAP-domain ASP, as the search model. The correct MR solution could only be obtained by truncating the polyalanine model of Na-ASP-2 and removing several loops. The structure reveals two CAP domains linked by an extended loop. Overall, the carboxyl-terminal CAP domain is more similar to Na-ASP-2 than to the amino-terminal CAP domain. A large central cavity extends from the amino-terminal CAP domain to the carboxyl-terminal CAP domain, encompassing the putative CAP-binding cavity. The putative CAP-binding cavity is a characteristic cavity in the carboxyl-terminal CAP domain that contains a His and Glu pair. These residues are conserved in all single-CAP-domain proteins, but are absent in the amino-terminal CAP domain. The conserved His residues are oriented such that they appear to be capable of directly coordinating a zinc ion as observed for CAP proteins from reptile venoms. This first structure of a two-CAP-domain ASP can serve as a template for homology modeling of other two-CAP-domain proteins.
Organizational Affiliation:
Pathology and Microbiology Department, 986495 Nebraska Medical Center, Omaha, NE 68198-6495, USA. oasojo@unmc.edu