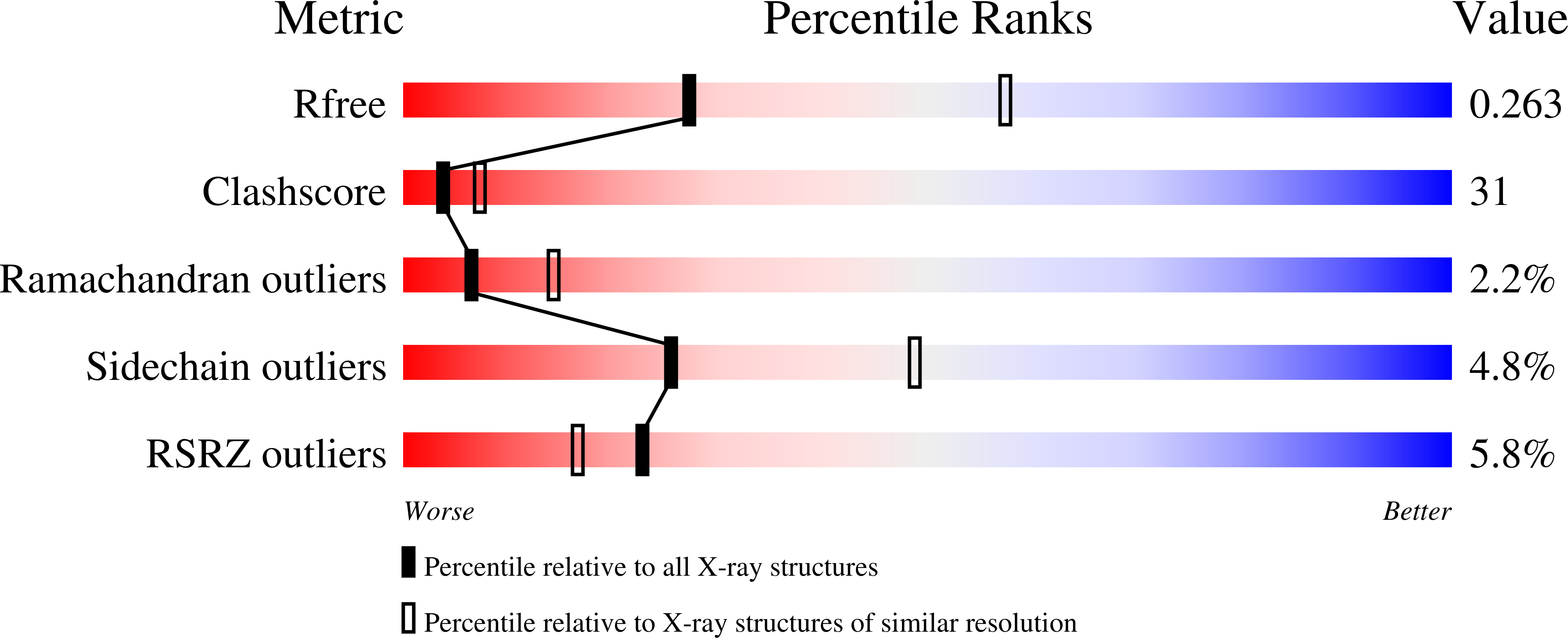
Structural study of TcaR and its complexes with multiple antibiotics from Staphylococcus epidermidis.
Chang, Y.M., Jeng, W.Y., Ko, T.P., Yeh, Y.J., Chen, C.K., Wang, A.H.(2010) Proc Natl Acad Sci U S A 107: 8617-8622
- PubMed: 20421503
- DOI: https://doi.org/10.1073/pnas.0913302107
- Primary Citation of Related Structures:
3KP2, 3KP3, 3KP4, 3KP5, 3KP6, 3KP7 - PubMed Abstract:
TcaR and IcaR are a weak and a strong negative regulator of transcription of the ica locus, respectively, and their presence prevents the poly-N-acetylglucosamine production and biofilm formation in Staphylococcus epidermidis. Although TcaR was shown to interact with the ica promoter, the precise binding region and the mechanism of interaction remained unclear. Here we present the 3D structure of TcaR in its apo form and in complex with salicylate as well as several aminoglycoside and beta-lactam antibiotics. A comparison of the native and complex TcaR structures indicates that the mechanism of regulation involves a large conformational change in the DNA-binding lobe. Here, we deduced the consensus binding sequence of two [ approximately TTNNAA] hexamers embedded in a 16 bp sequence for a TcaR dimer. Six TcaR dimers bind specifically to three approximately 33 bp segments close to the IcaR binding region with varying affinities, and their repressor activity is directly interfered by salicylate and different classes of natural antimicrobial compounds. We also found in this study that the antimicrobial compounds we tested were shown not only to inhibit TcaR-DNA interaction but also to further induce biofilm formation in S. epidermidis in our in vivo assay. The results support a general mechanism for antibiotics in regulating TcaR-DNA interaction and thereby help understand the effect of antibiotic exposure on bacterial antibiotic resistance through biofilm formation.
Organizational Affiliation:
Institute of Biochemical Sciences, National Taiwan University, Taipei 106, Taiwan.