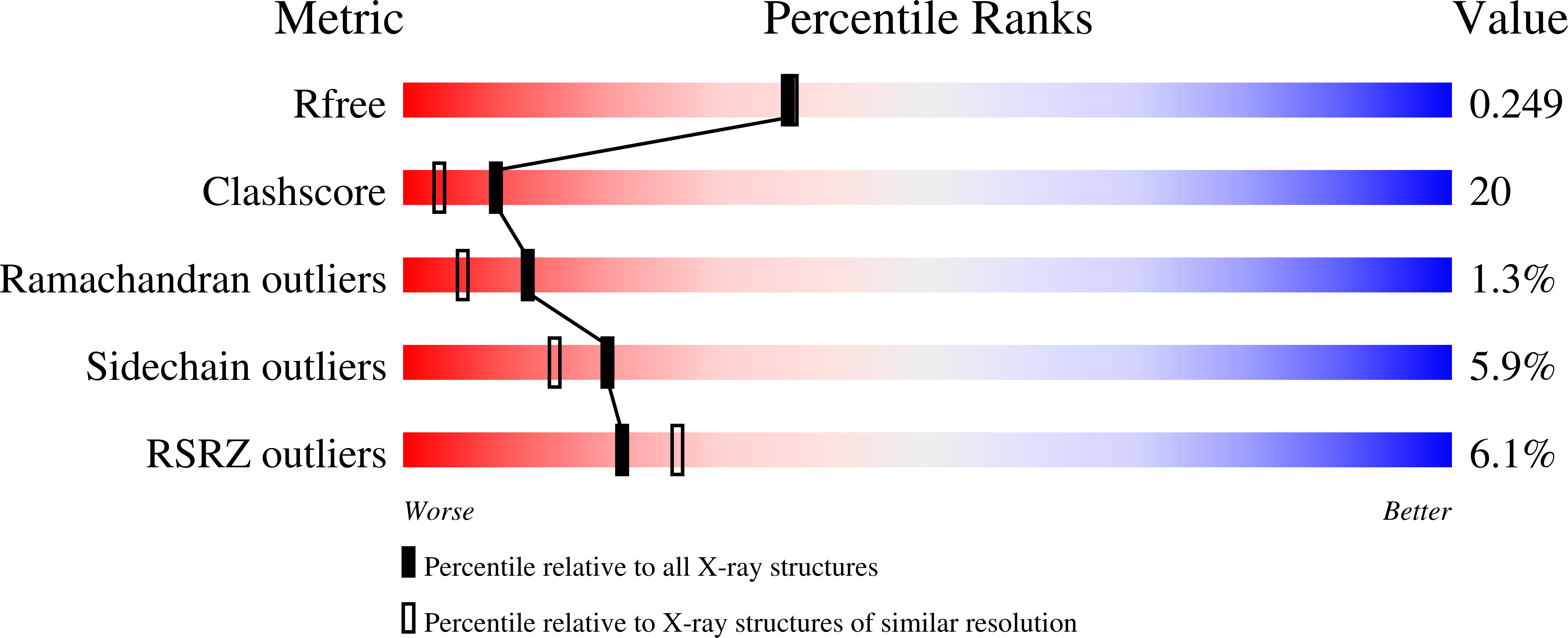
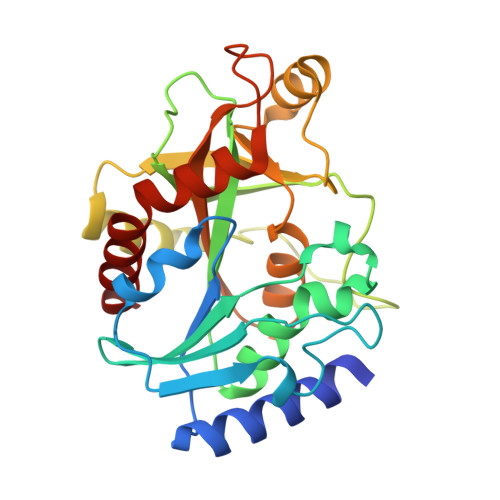
Crystallographic and docking studies of purine nucleoside phosphorylase from Mycobacterium tuberculosis.
Ducati, R.G., Basso, L.A., Santos, D.S., de Azevedo, W.F.(2010) Bioorg Med Chem 18: 4769-4774
- PubMed: 20570524
- DOI: https://doi.org/10.1016/j.bmc.2010.05.009
- Primary Citation of Related Structures:
3IOM - PubMed Abstract:
This work describes for the first time the structure of purine nucleoside phosphorylase from Mycobacterium tuberculosis (MtPNP) in complex with sulfate and its natural substrate, 2'-deoxyguanosine, and its application to virtual screening. We report docking studies of a set of molecules against this structure. Application of polynomial empirical scoring function was able to rank docking solutions with good predicting power which opens the possibility to apply this new criterion to analyze docking solutions and screen small-molecule databases for new chemical entities to inhibit MtPNP.
Organizational Affiliation:
Centro de Pesquisas em Biologia Molecular e Funcional, Instituto de Pesquisas Biomédicas, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Avenida Ipiranga 6681/92-A, 90619-900 Porto Alegre, RS, Brazil.