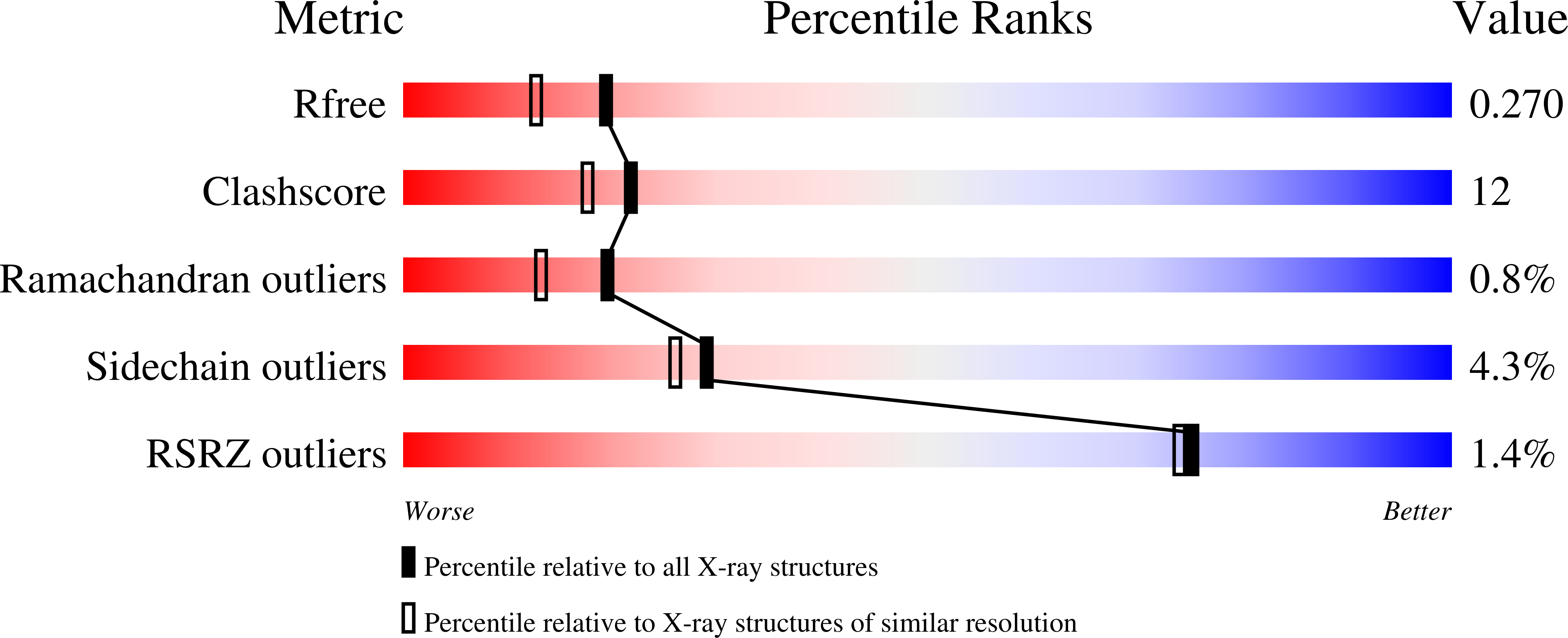
CcpA from Geobacter sulfurreducens is a basic di-heme cytochrome c peroxidase.
Hoffmann, M., Seidel, J., Einsle, O.(2009) J Mol Biol 393: 951-965
- PubMed: 19735665
- DOI: https://doi.org/10.1016/j.jmb.2009.09.001
- Primary Citation of Related Structures:
3HQ6, 3HQ7, 3HQ8, 3HQ9 - PubMed Abstract:
Bacterial di-heme cytochrome c peroxidases (CcpAs) protect the cell from reactive oxygen species by reducing hydrogen peroxide to water. The enzymes are c-type cytochromes, with both heme groups covalently attached to the protein chain via a characteristic binding motif. The genome of the dissimilatory metal-reducing bacterium Geobacter sulfurreducens revealed the presence of a ccpA gene and we isolated the gene product after recombinant expression in Escherichia coli. CcpA from G. sulfurreducens exhibited in vitro peroxidase activity with ABTS(2-) [2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)] as an electron donor, and the three-dimensional structure of the dimeric enzyme has been determined to high resolution. For activation, CcpA commonly requires reduction, with the exception of the Nitrosomonas europaea enzyme that retains its activity in the oxidized state. A G94K/K97Q/R100I triple point mutant was created to mimic the critical loop region of N. europaea CcpA, but its crystal structure revealed that the inactive, bis-histidinyl-coordinated form of the active-site heme group was retained. Subsequent mutational studies thus addressed an adjacent loop region, where a change in secondary structure accompanies the reductive activation of the enzyme. While an A124K/K128A double mutant did not show significant changes, the CcpA variants S134P/V135K and S134P led to a distortion of the loop region, accompanied by an opening of the active-site loop, leaving the enzyme in a constitutively active state.
Organizational Affiliation:
Lehrstuhl für Biochemie, Institut für organische Chemie und Biochemie, Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, Freiburg 79104, Germany.