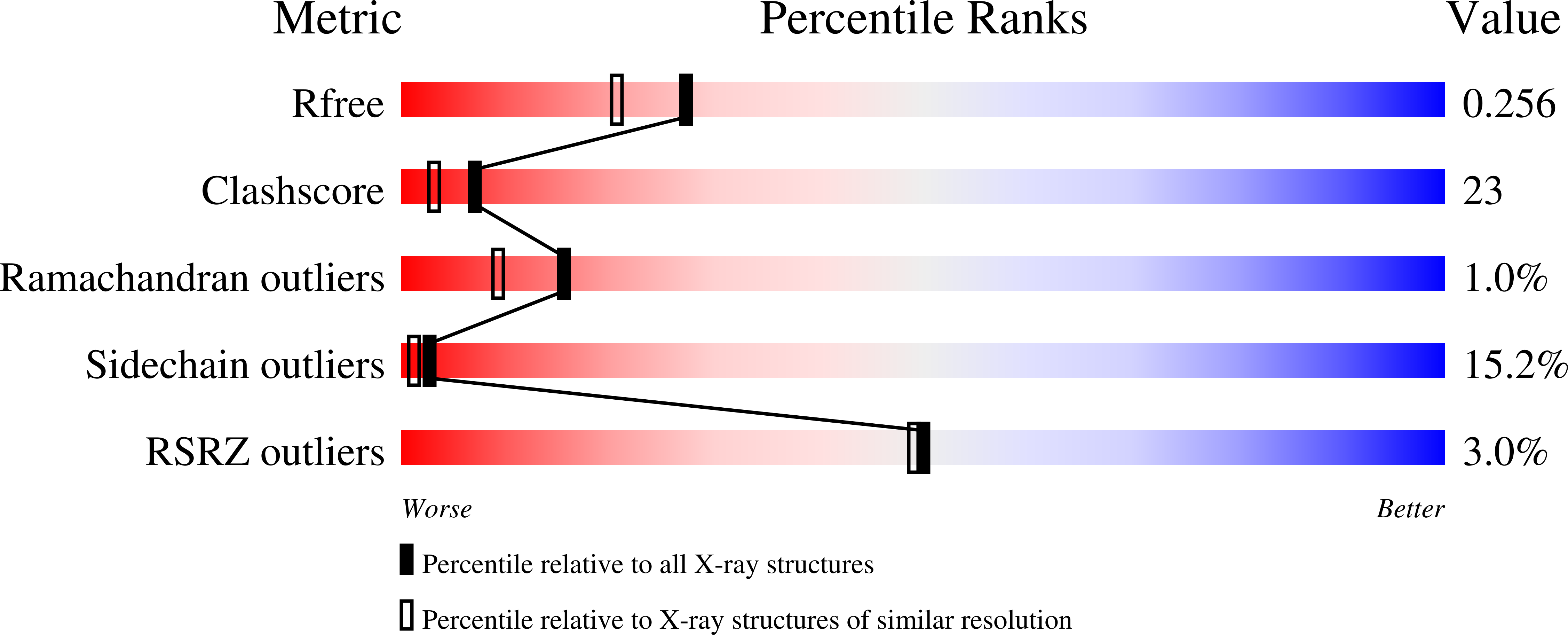
X-ray structure and kinetic properties of ornithine transcarbamoylase from the human parasite Giardia lamblia.
Galkin, A., Kulakova, L., Wu, R., Gong, M., Dunaway-Mariano, D., Herzberg, O.(2009) Proteins 76: 1049-1053
- PubMed: 19507245
- DOI: https://doi.org/10.1002/prot.22469
- Primary Citation of Related Structures:
3GRF
Organizational Affiliation:
W.M. Keck Laboratory for Structural Biology, Center for Advanced Research in Biotechnology, University of Maryland Biotechnology Institute, Rockville, Maryland, USA.