Crystal Structure of Mouse Mitochondrial Thioredoxin Reductase, C-terminal 3-residue truncation
Eckenroth, B.E., Hondal, R.J., Everse, S.J.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Thioredoxin reductase 2 | 488 | Mus musculus | Mutation(s): 0 Gene Names: Txnrd2, Trxr2 EC: 1.8.1.9 | 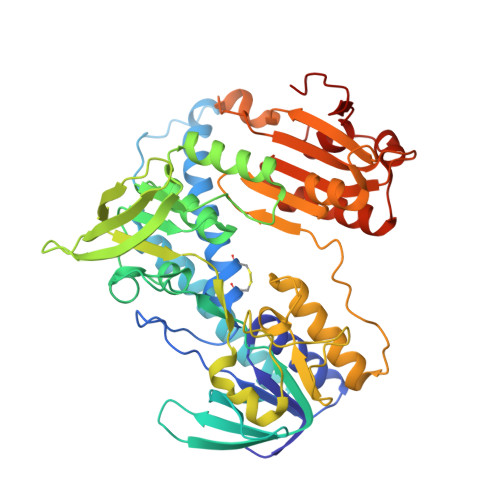 | |
UniProt | |||||
Find proteins for Q9JLT4 (Mus musculus) Explore Q9JLT4 Go to UniProtKB: Q9JLT4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9JLT4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| FAD Query on FAD | B [auth A] | FLAVIN-ADENINE DINUCLEOTIDE C27 H33 N9 O15 P2 VWWQXMAJTJZDQX-UYBVJOGSSA-N |  | ||
| NA7 Query on NA7 | C [auth A] | [(2R,3R,4R,5R)-5-(6-AMINO-9H-PURIN-9-YL)-3-HYDROXY-4-(PHOSPHONOOXY)TETRAHYDROFURAN-2-YL]METHYL [(2R,3S,4S)-3,4-DIHYDROXYTETRAHYDROFURAN-2-YL]METHYL DIHYDROGEN DIPHOSPHATE C15 H24 N5 O16 P3 XDDBFIXGEVGCEU-AOOZFPJJSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 110.507 | α = 90 |
| b = 110.507 | β = 90 |
| c = 208.025 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| MOLREP | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| MAR345dtb | data collection |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |