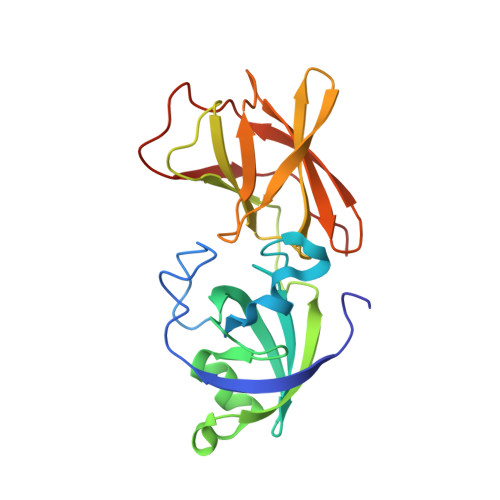
Crystal structure and activity of Bacillus subtilis YoaJ (EXLX1), a bacterial expansin that promotes root colonization.
Kerff, F., Amoroso, A., Herman, R., Sauvage, E., Petrella, S., Filee, P., Charlier, P., Joris, B., Tabuchi, A., Nikolaidis, N., Cosgrove, D.J.(2008) Proc Natl Acad Sci U S A 105: 16876-16881
- PubMed: 18971341
- DOI: https://doi.org/10.1073/pnas.0809382105
- Primary Citation of Related Structures:
2BH0, 3D30 - PubMed Abstract:
We solved the crystal structure of a secreted protein, EXLX1, encoded by the yoaJ gene of Bacillus subtilis. Its structure is remarkably similar to that of plant beta-expansins (group 1 grass pollen allergens), consisting of 2 tightly packed domains (D1, D2) with a potential polysaccharide-binding surface spanning the 2 domains. Domain D1 has a double-psi beta-barrel fold with partial conservation of the catalytic site found in family 45 glycosyl hydrolases and in the MltA family of lytic transglycosylases. Domain D2 has an Ig-like fold similar to group 2/3 grass pollen allergens, with structural features similar to a type A carbohydrate-binding domain. EXLX1 bound to plant cell walls, cellulose, and peptidoglycan, but it lacked lytic activity against a variety of plant cell wall polysaccharides and peptidoglycan. EXLX1 promoted plant cell wall extension similar to, but 10 times weaker than, plant beta-expansins, which synergistically enhanced EXLX1 activity. Deletion of the gene encoding EXLX1 did not affect growth or peptidoglycan composition of B. subtilis in liquid medium, but slowed lysis upon osmotic shock and greatly reduced the ability of the bacterium to colonize maize roots. The presence of EXLX1 homologs in a small but diverse set of plant pathogens further supports a role in plant-bacterial interactions. Because plant expansins have proved difficult to express in active form in heterologous systems, the discovery of a bacterial homolog opens the door for detailed structural studies of expansin function.
Organizational Affiliation:
Université de Liège, Centre d'Ingénierie des Protéines, Institut de Chimie B6, 4000 Liège, Belgium.