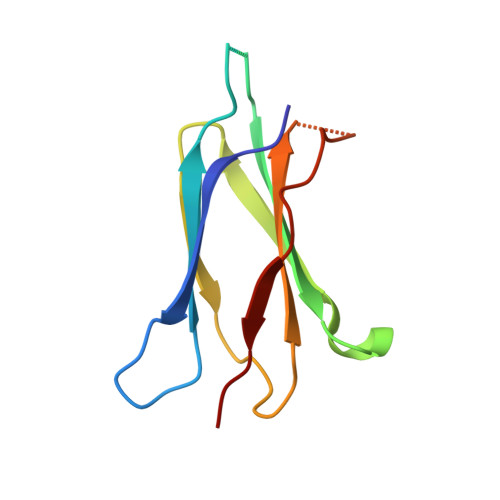
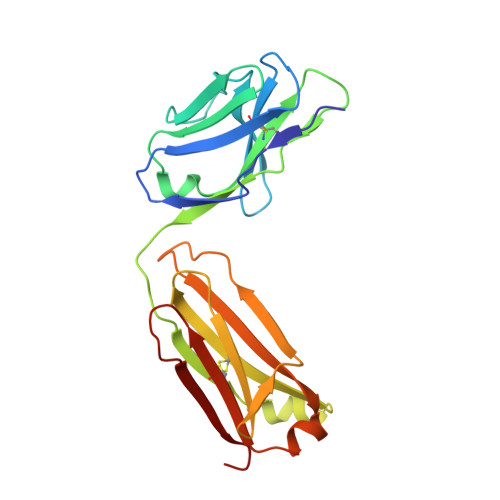
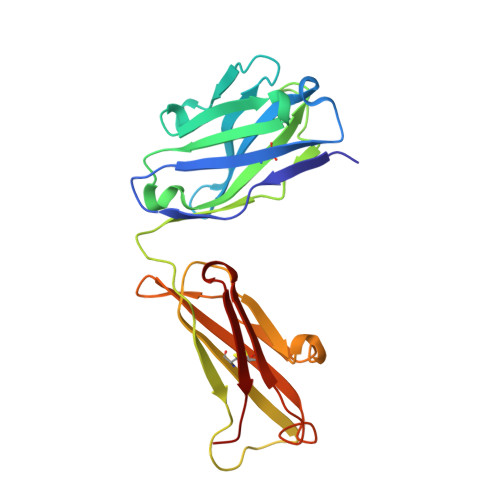
Structural features of interfacial tyrosine residue in ROBO1 fibronectin domain-antibody complex: Crystallographic, thermodynamic, and molecular dynamic analyses
Nakayama, T., Mizohata, E., Yamashita, T., Nagatoishi, S., Nakakido, M., Iwanari, H., Mochizuki, Y., Kado, Y., Yokota, Y., Satoh, R., Tsumoto, K., Fujitani, H., Kodama, T., Hamakubo, T., Inoue, T.(2015) Protein Sci 24: 328-340
- PubMed: 25492858
- DOI: https://doi.org/10.1002/pro.2619
- Primary Citation of Related Structures:
3WIH, 3WII - PubMed Abstract:
ROBO1, fibronectin Type-III domain (Fn)-containing protein, is a novel immunotherapeutic target for hepatocellular carcinoma in humans. The crystal structure of the antigen-binding fragment (Fab) of B2212A, the monoclonal antibody against the third Fn domain (Fn3) of ROBO1, was determined in pursuit of antibody drug for hepatocellular carcinoma. This effort was conducted in the presence or absence of the antigen, with the chemical features being investigated by determining the affinity of the antibody using molecular dynamics (MD) and thermodynamics. The structural comparison of B2212A Fab between the complex and the free form revealed that the interfacial Tyr(L) 50 (superscripts L, H, and F stand for the residues in the light chain, heavy chain, and Fn3, respectively) played important roles in Fn3 recognition. That is, the aromatic ring of Tyr(L) 50 pivoted toward Phe(F) 68, forming a CH/π interaction and a new hydrogen bond with the carbonyl O atom of Phe(F) 68. MD simulations predicted that the Tyr(L) 50-Phe(F) 68 interaction almost entirely dominated Fab-Fn3 binding, and Ala-substitution of Tyr(L) 50 led to a reduced binding of the resultant complex. On the contrary, isothermal titration calorimetry experiments underscored that Ala-substitution of Tyr(L) 50 caused an increase of the binding enthalpy between B2212A and Fn3, but importantly, it induced an increase of the binding entropy, resulting in a suppression of loss in the Gibbs free energy in total. These results suggest that mutation analysis considering the binding entropy as well as the binding enthalpy will aid in the development of novel antibody drugs for hepatocellular carcinoma.
Organizational Affiliation:
Structural Physical Chemistry, Division of Applied Chemistry, Graduate School of Engineering, Osaka University, Osaka, Japan.