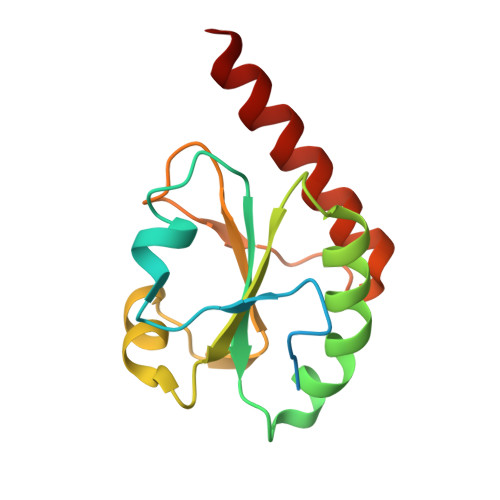
Synergistic cooperation of PDI family members in peroxiredoxin 4-driven oxidative protein folding
Sato, Y., Kojima, R., Okumura, M., Hagiwara, M., Masui, S., Maegawa, K., Saiki, M., Horibe, T., Suzuki, M., Inaba, K.(2013) Sci Rep 3: 2456-2456
- PubMed: 23949117
- DOI: https://doi.org/10.1038/srep02456
- Primary Citation of Related Structures:
3VWU, 3VWV, 3VWW, 3W8J - PubMed Abstract:
The mammalian endoplasmic reticulum (ER) harbors disulfide bond-generating enzymes, including Ero1α and peroxiredoxin 4 (Prx4), and nearly 20 members of the protein disulfide isomerase family (PDIs), which together constitute a suitable environment for oxidative protein folding. Here, we clarified the Prx4 preferential recognition of two PDI family proteins, P5 and ERp46, and the mode of interaction between Prx4 and P5 thioredoxin domain. Detailed analyses of oxidative folding catalyzed by the reconstituted Prx4-PDIs pathways demonstrated that, while P5 and ERp46 are dedicated to rapid, but promiscuous, disulfide introduction, PDI is an efficient proofreader of non-native disulfides. Remarkably, the Prx4-dependent formation of native disulfide bonds was accelerated when PDI was combined with ERp46 or P5, suggesting that PDIs work synergistically to increase the rate and fidelity of oxidative protein folding. Thus, the mammalian ER seems to contain highly systematized oxidative networks for the efficient production of large quantities of secretory proteins.
Organizational Affiliation:
1] Division of Protein Chemistry, Medical Institute of Bioregulation, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan [2].