Quasiracemic crystallization as a tool to assess the accommodation of noncanonical residues in nativelike protein conformations.
Mortenson, D.E., Satyshur, K.A., Guzei, I.A., Forest, K.T., Gellman, S.H.(2012) J Am Chem Soc 134: 2473-2476
- PubMed: 22280019
- DOI: https://doi.org/10.1021/ja210045s
- Primary Citation of Related Structures:
3TJW, 3TRV, 3TRW, 3TRY - PubMed Abstract:
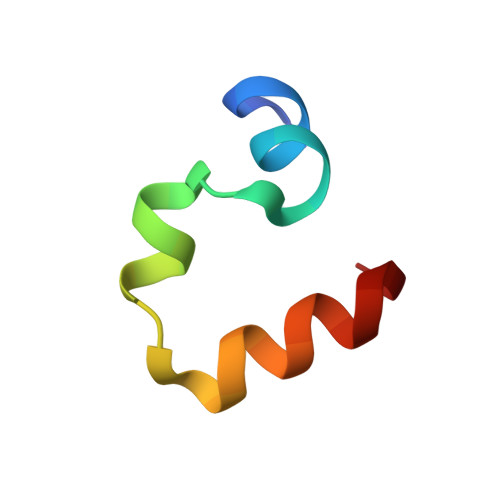
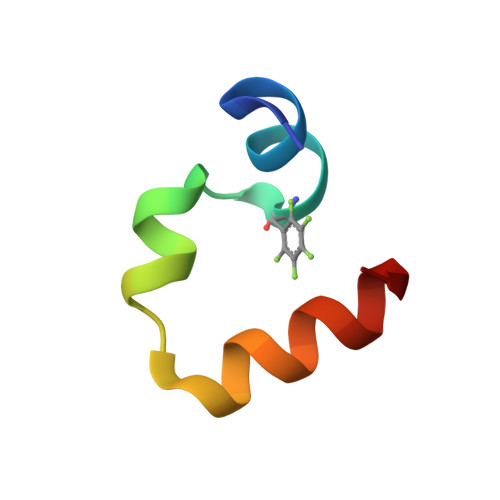
Quasiracemic crystallization has been used to obtain high-resolution structures of two variants of the villin headpiece subdomain (VHP) that contain a pentafluorophenylalanine (F(5)Phe) residue in the hydrophobic core. In each case, the crystal contained the variant constructed from l-amino acids and the native sequence constructed from d-amino acids. We were motivated to undertake these studies by reports that racemic proteins crystallize more readily than homochiral forms and the prospect that quasiracemic crystallization would enable us to determine whether a polypeptide containing a noncanonical residue can closely mimic the tertiary structure of the native sequence. The results suggest that quasiracemic crystallization may prove to be generally useful for assessing mimicry of naturally evolved protein folding patterns by polypeptides that contain unnatural side-chain or backbone subunits.
Organizational Affiliation:
Department of Chemistry, University of Wisconsin, Madison, Wisconsin 53706, USA.