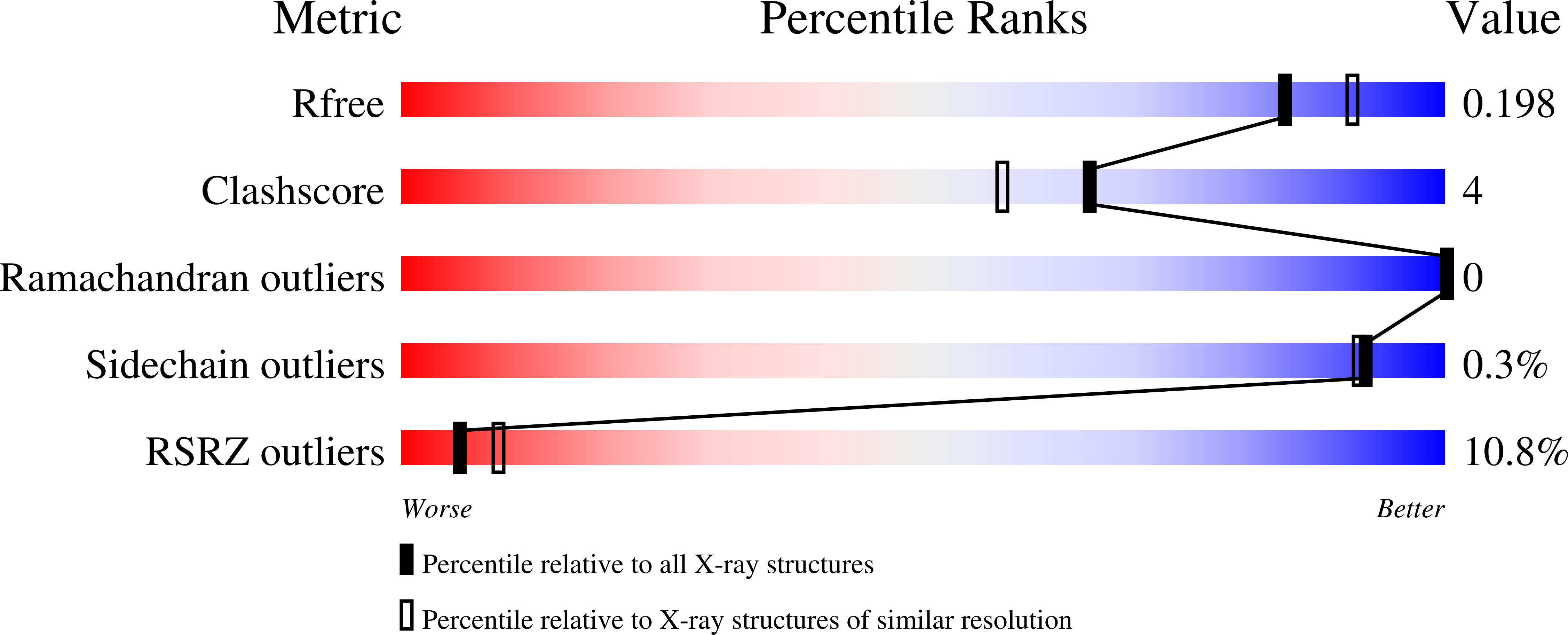
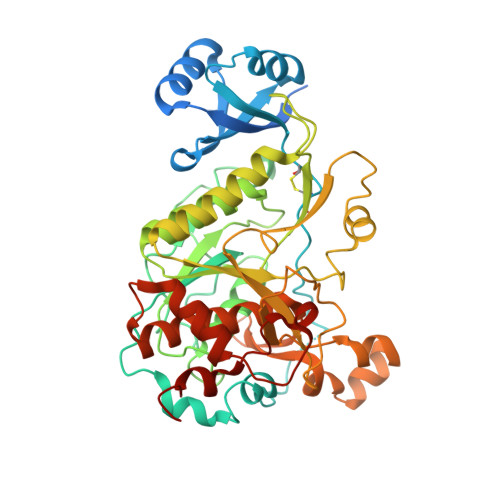
1.95 Angstrom Resolution Crystal Structure of Epidermin Leader Peptide Processing Serine Protease (EpiP) S393A Mutant from Staphylococcus aureus.
Minasov, G., Kuhn, M., Ruan, J., Halavaty, A., Shuvalova, L., Dubrovska, I., Winsor, J., Bagnoli, F., Falugi, F., Bottomley, M., Grandi, G., Anderson, W.F., Center for Structural Genomics of Infectious Diseases (CSGID)To be published.