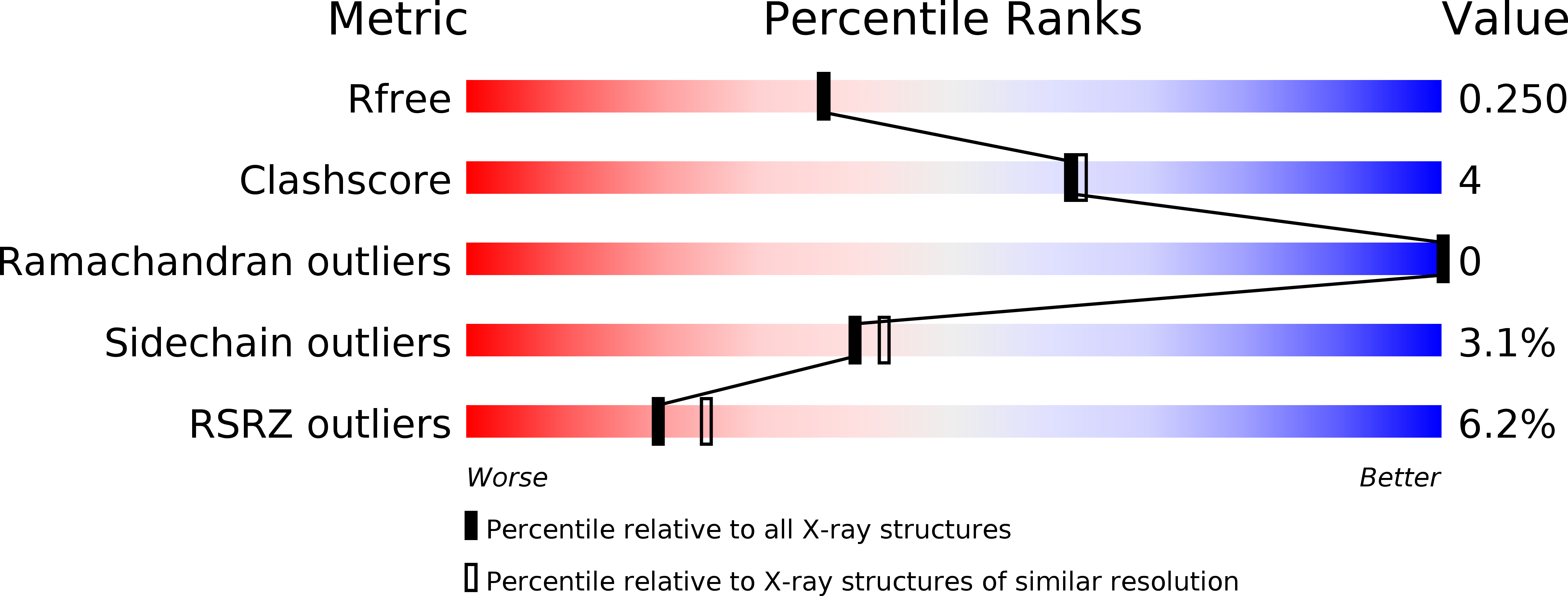
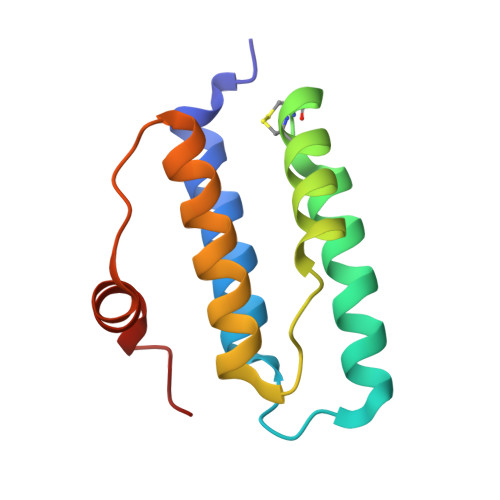
Dimer interface migration in a viral sulfhydryl oxidase
Hakim, M., Fass, D.(2009) J Mol Biol 391: 758-768
- PubMed: 19576902
- DOI: https://doi.org/10.1016/j.jmb.2009.06.070
- Primary Citation of Related Structures:
3GWL, 3GWN - PubMed Abstract:
Large double-stranded DNA viruses, including poxviruses and mimiviruses, encode enzymes to catalyze the formation of disulfide bonds in viral proteins produced in the cell cytosol, an atypical location for oxidative protein folding. These viral disulfide catalysts belong to a family of sulfhydryl oxidases that are dimers of a small five-helix fold containing a Cys-X-X-Cys motif juxtaposed to a flavin adenine dinucleotide cofactor. We report that the sulfhydryl oxidase pB119L from African swine fever virus (ASFV) uses for self-assembly surface different from that observed in homologs from mammals, plants, and fungi. Within a protein family, different packing interfaces for the same oligomerization state are extremely rare. We find that the alternate dimerization mode seen in ASFV pB119L is not characteristic of all viral sulfhydryl oxidases, as the flavin-binding domain from a mimivirus sulfhydryl oxidase assumes the same dimer structure as the known eukaryotic enzymes. ASFV pB119L demonstrates the potential of large double-stranded DNA viruses, which have faster mutation rates than their hosts and the tendency to incorporate host genes, to pioneer new protein folds and self-assembly modes.
Organizational Affiliation:
Department of Structural Biology, Weizmann Institute of Science, Rehovot, Israel.