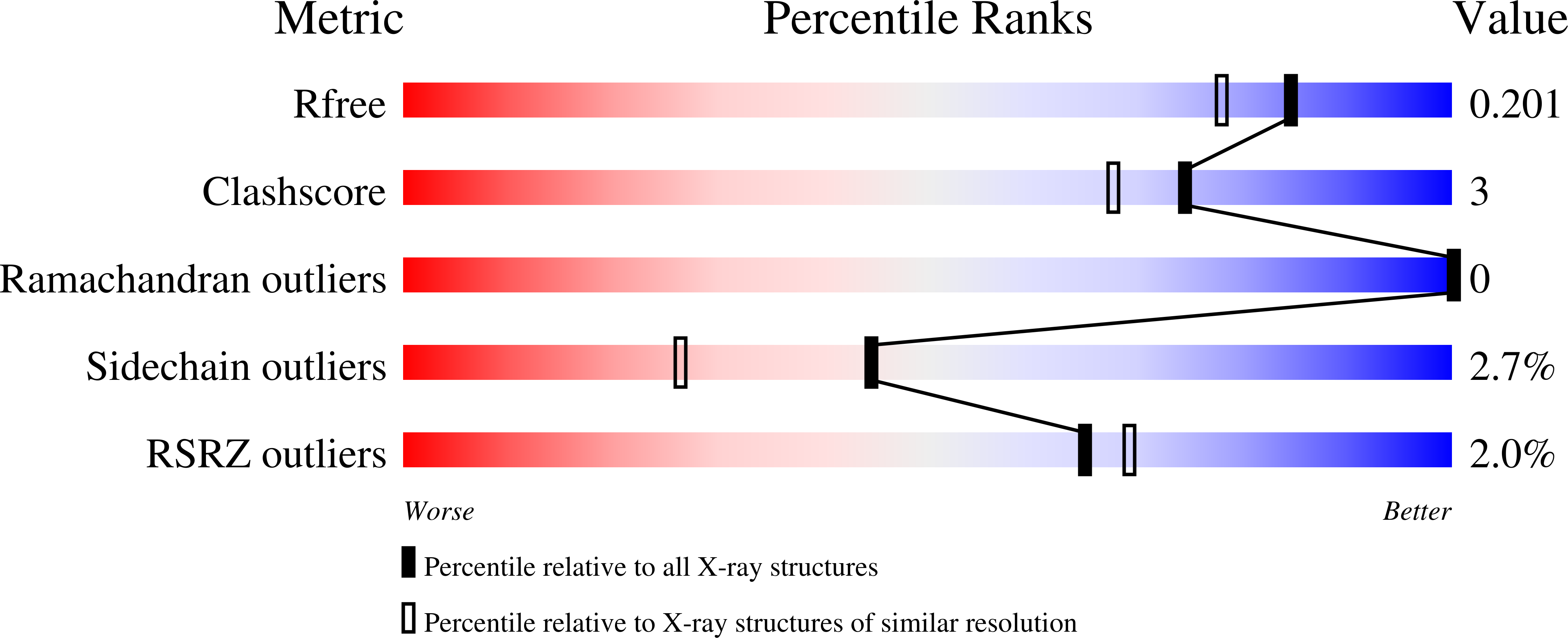
Oxygen pressurized X-ray crystallography: probing the dioxygen binding site in cofactorless urate oxidase and implications for its catalytic mechanism.
Colloc'h, N., Gabison, L., Monard, G., Altarsha, M., Chiadmi, M., Marassio, G., Sopkova-de Oliveira Santos, J., El Hajji, M., Castro, B., Abraini, J.H., Prange, T.(2008) Biophys J 95: 2415-2422
- PubMed: 18375516
- DOI: https://doi.org/10.1529/biophysj.107.122184
- Primary Citation of Related Structures:
2ZKA, 2ZKB, 3CKS, 3CKU - PubMed Abstract:
The localization of dioxygen sites in oxygen-binding proteins is a nontrivial experimental task and is often suggested through indirect methods such as using xenon or halide anions as oxygen probes. In this study, a straightforward method based on x-ray crystallography under high pressure of pure oxygen has been developed. An application is given on urate oxidase (UOX), a cofactorless enzyme that catalyzes the oxidation of uric acid to 5-hydroxyisourate in the presence of dioxygen. UOX crystals in complex with a competitive inhibitor of its natural substrate are submitted to an increasing pressure of 1.0, 2.5, or 4.0 MPa of gaseous oxygen. The results clearly show that dioxygen binds within the active site at a location where a water molecule is usually observed but does not bind in the already characterized specific hydrophobic pocket of xenon. Moreover, crystallizing UOX in the presence of a large excess of chloride (NaCl) shows that one chloride ion goes at the same location as the oxygen. The dioxygen hydrophilic environment (an asparagine, a histidine, and a threonine residues), its absence within the xenon binding site, and its location identical to a water molecule or a chloride ion suggest that the dioxygen site is mainly polar. The implication of the dioxygen location on the mechanism is discussed with respect to the experimentally suggested transient intermediates during the reaction cascade.
Organizational Affiliation:
CI-NAPS, UMR 6232-UCBN-CNRS-CEA, Centre Cyceron, 14074 Caen cedex, France. colloch@cyceron.fr