Synthesis and Biochemical Evaluation of Selective Inhibitors of Class II Fructose Bisphosphate Aldolases: Towards New Synthetic Antibiotics.
Fonvielle, M., Coincon, M., Daher, R., Desbenoit, N., Kosieradzka, K., Barilone, N., Gicquel, B., Sygusch, J., Jackson, M., Therisod, M.(2008) Chemistry 14: 8521-8529
- PubMed: 18688832
- DOI: https://doi.org/10.1002/chem.200800857
- Primary Citation of Related Structures:
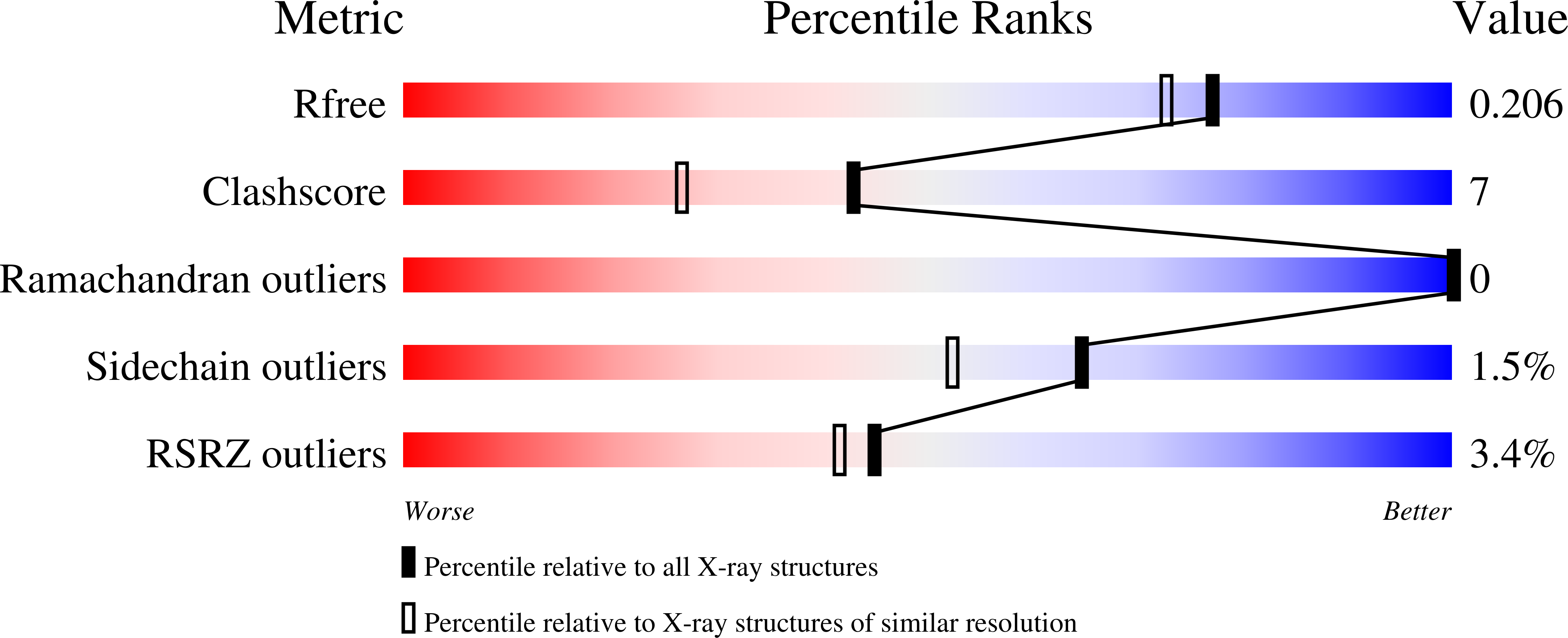
3C4U, 3C52, 3C56 - PubMed Abstract:
We report the synthesis and biochemical evaluation of selective inhibitors of class II (zinc-dependent) fructose bisphosphate aldolases. The most active compound is a simplified analogue of fructose bisphosphate, bearing a well-positioned metal chelating group. It is a powerful and highly selective competitive inhibitor of isolated class II aldolases. We report crystallographic studies of this inhibitor bound in the active site of the Helicobacter pylori enzyme. The compound also shows activity against Mycobacterium tuberculosis isolates.
Organizational Affiliation:
LCBB-ICMMO, UMR 8182, Université Paris-Sud, Orsay, France.