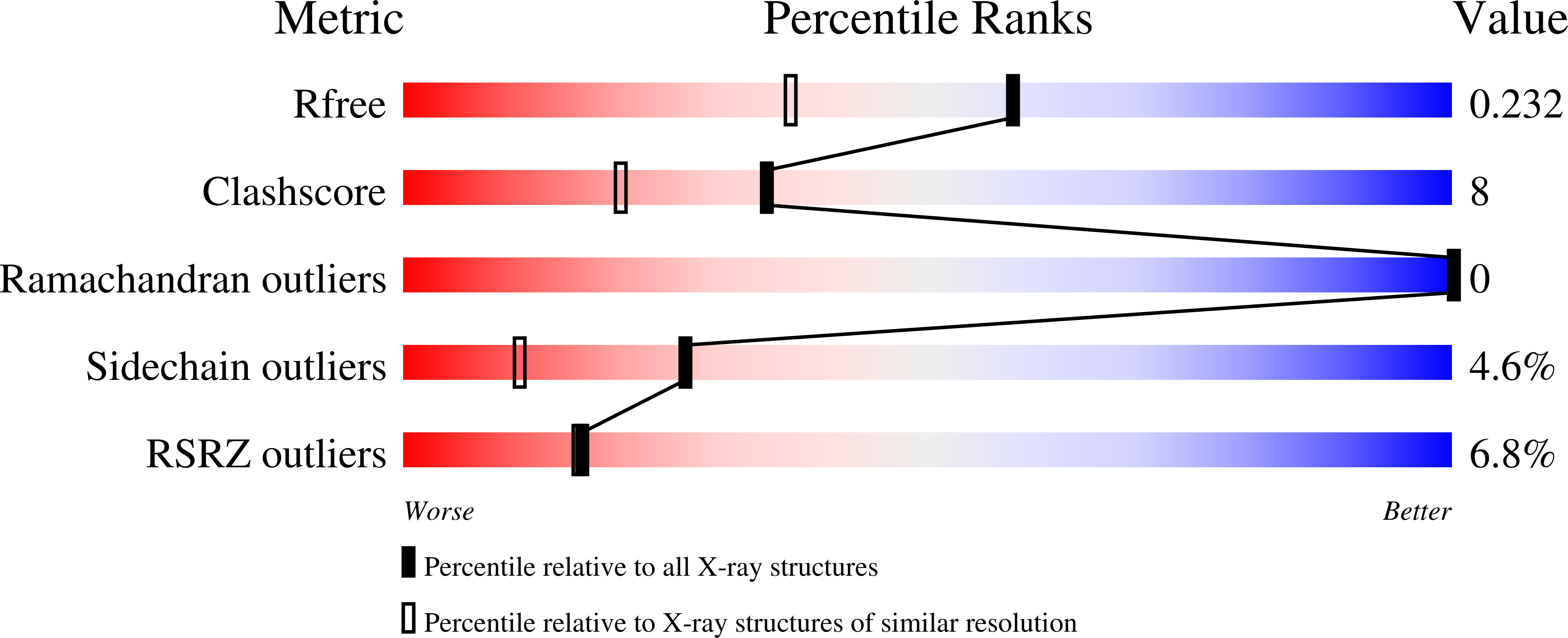
A hydrogen-bonding network formed by the B10-E7-E11 residues of a truncated hemoglobin from Tetrahymena pyriformis is critical for stability of bound oxygen and nitric oxide detoxification.
Igarashi, J., Kobayashi, K., Matsuoka, A.(2011) J Biol Inorg Chem 16: 599-609
- PubMed: 21298303
- DOI: https://doi.org/10.1007/s00775-011-0761-3
- Primary Citation of Related Structures:
3AQ5, 3AQ6, 3AQ7, 3AQ8, 3AQ9 - PubMed Abstract:
Truncated hemoglobins (trHbs) are distributed from bacteria to unicellular eukaryotes and have roles in oxygen transport and nitric oxide detoxification. It is known that trHbs exist in ciliates of the Tetrahymena group, but trHb structure and function remain poorly understood. To investigate trHb function with respect to stability of bound oxygen and protein structure, we measured the oxygen binding kinetics of Tetrahymena pyriformis trHb, and determined the crystal structure of the protein. The O(2) association and dissociation rate constants of T. pyriformis trHb were 5.5 μM(-1 )s(-1) and 0.18 s(-1), respectively. The autooxidation rate constant was 3.8 × 10(-3) h(-1). These values are similar to those of HbN from Mycobacterium tuberculosis. The three-dimensional structure of an Fe(II)-O(2) complex of T. pyriformis trHb was determined at 1.73-Å resolution. Tyr25 (B10) and Gln46 (E7) were hydrogen-bonded to a heme-bound O(2) molecule. Tyr25 donated a hydrogen bond to the terminal oxygen atom, whereas Gln46 hydrogen-bonded to the proximal oxygen atom. Furthermore, Tyr25 was hydrogen-bonded to the Gln46 and Gln50 (E11) residues. Mutations at Tyr25, Gln46, and Gln50 increased the O(2) dissociation and autooxidation rate constants. An Fe(III)-H(2)O complex of T. pyriformis trHb was formed following reaction of the Fe(II)-O(2) complex of T. pyriformis trHb, in a crystal state, with nitric oxide. This suggests that T. pyriformis trHb functions in nitric oxide detoxification.
Organizational Affiliation:
Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai, Japan. jotaro@tagen.tohoku.ac.jp