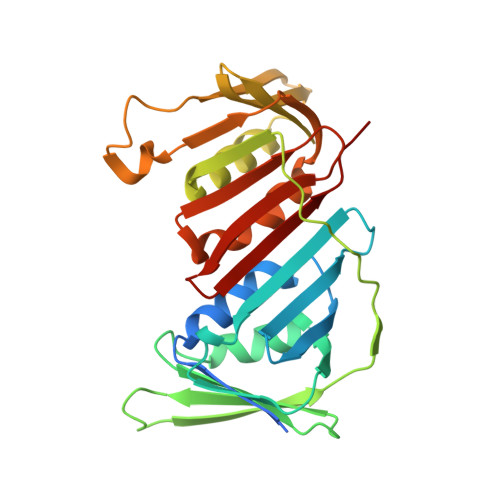
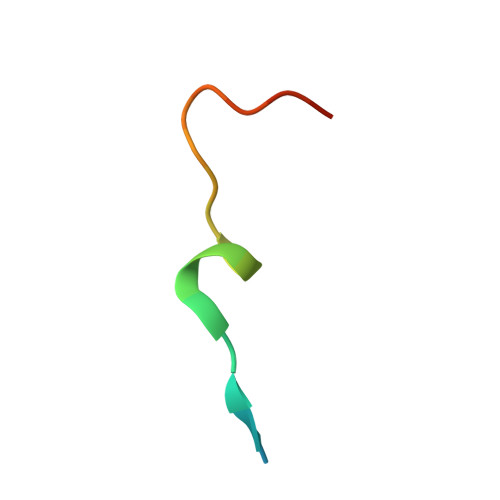
Crystal structures of the Arabidopsis thaliana proliferating cell nuclear antigen 1 and 2 proteins complexed with the human p21 C-terminal segment
Strzalka, W., Oyama, T., Tori, K., Morikawa, K.(2009) Protein Sci 18: 1072-1080
- PubMed: 19388052
- DOI: https://doi.org/10.1002/pro.117
- Primary Citation of Related Structures:
2ZVV, 2ZVW - PubMed Abstract:
The proliferating cell nuclear antigen (PCNA) is well recognized as one of the essential cellular components of the DNA replication machinery in all eukaryotic organisms. Despite their prominent importance, very little biochemical and structural information about plant PCNAs is available, in comparison with that obtained from other eukaryotic organisms. We have determined the atomic resolution crystal structures of the two distinct Arabidopsis thaliana PCNAs (AtPCNA), both complexed with the C-terminal segment of human p21. Both AtPCNAs form homotrimeric ring structures, which are essentially identical to each other, including the major contacts with the p21 peptide. The structure of the amino-terminal half of the p21 peptide, containing the typical PIP box sequence, is remarkably similar to those observed in the previously reported crystal structures of the human and archaeal PCNA-PIP box complexes. Meanwhile, the carboxy-terminal halves of the p21 peptide in the plant PCNA complexes are bound to the protein in a unique manner, most probably because of crystal packing effects. A surface plasmon resonance analysis revealed high affinity between each AtPCNA and the C-terminal fragment of human p21. This result strongly suggests that the interaction is functionally significant, although no plant homologs of p21 have been identified yet. We also discovered that AtPCNA1 and AtPCNA2 form heterotrimers, implying that hetero-PCNA rings may play critical roles in cellular signal transduction, particularly in DNA repair.
Organizational Affiliation:
Department of Molecular Genetics, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, 30-387 Krakow, Poland.