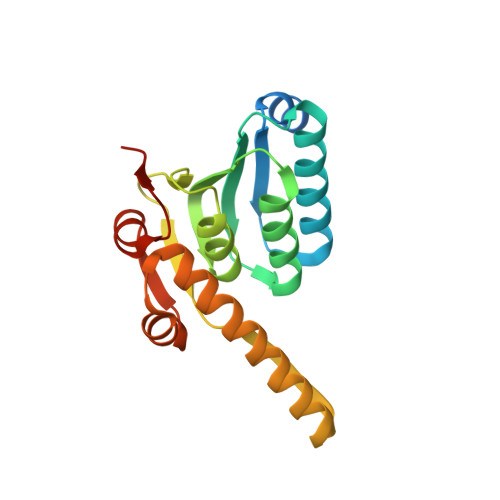
The structural basis for the activation and peptide recognition of bacterial ClpP
Kim, D.Y., Kim, K.K.(2008) J Mol Biol 379: 760-771
- PubMed: 18468623
- DOI: https://doi.org/10.1016/j.jmb.2008.04.036
- Primary Citation of Related Structures:
2ZL0, 2ZL2, 2ZL3, 2ZL4 - PubMed Abstract:
ClpP and its ATPase compartment, ClpX or ClpA, remove misfolded proteins in cells and are of utmost importance in protein quality control. The ring hexamers of ClpA or ClpX recognize, unfold, and translocate target substrates into the degradation chamber of the double-ring tetradecamer of ClpP. The overall reaction scheme catalyzed by ClpXP or ClpAP has been proposed; however, the molecular mechanisms associated with substrate recognition and degradation have not yet been clarified in detail. To investigate these mechanisms, we determined the crystal structures of ClpP from Helicobacter pylori in complex with product peptides bound to the active site as well as in the apo state. In the complex structure, the peptides are zipped with two antiparallel strands of ClpP and point to the adjacent active site, thus providing structural explanations for the broad substrate specificity, the product inhibition and the processive degradation of substrates in the chamber. The structures also suggest that substrate binding causes local conformational changes around the active site that ultimately induce the active conformation of ClpP.
Organizational Affiliation:
The Department of Molecular Cell Biology, Institute of Basic Science, Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Suwon 440-746, Korea.