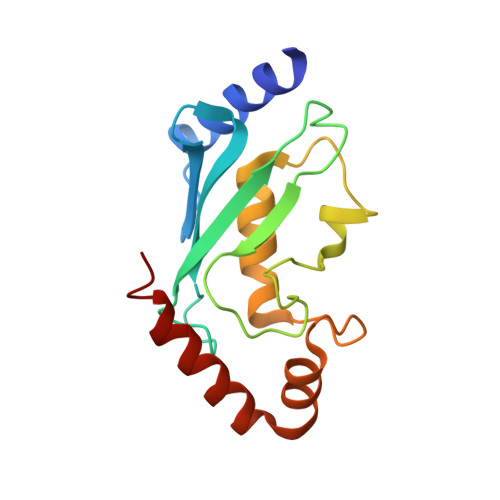
E3 Ligase Rad18 Promotes Monoubiquitination Rather Than Ubiquitin Chain Formation by E2 Enzyme Rad6.
Hibbert, R.G., Huang, A., Boelens, R., Sixma, T.K.(2011) Proc Natl Acad Sci U S A 108: 5590
- PubMed: 21422291
- DOI: https://doi.org/10.1073/pnas.1017516108
- Primary Citation of Related Structures:
2YB6, 2YBF - PubMed Abstract:
In ubiquitin conjugation, different combinations of E2 and E3 enzymes catalyse either monoubiquitination or ubiquitin chain formation. The E2/E3 complex Rad6/Rad18 exclusively monoubiquitinates the proliferating cell nuclear antigen (PCNA) to signal for "error prone" DNA damage tolerance, whereas a different set of conjugation enzymes is required for ubiquitin chain formation on PCNA. Here we show that human E2 enzyme Rad6b is intrinsically capable of catalyzing ubiquitin chain formation. This activity is prevented during PCNA ubiquitination by the interaction of Rad6 with E3 enzyme Rad18. Using NMR and X-ray crystallography we show that the R6BD of Rad18 inhibits this activity by competing with ubiquitin for a noncovalent "backside" binding site on Rad6. Our findings provide mechanistic insights into how E3 enzymes can regulate the ubiquitin conjugation process.
Organizational Affiliation:
Division of Biochemistry and Center for Biomedical Genetics, Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands.