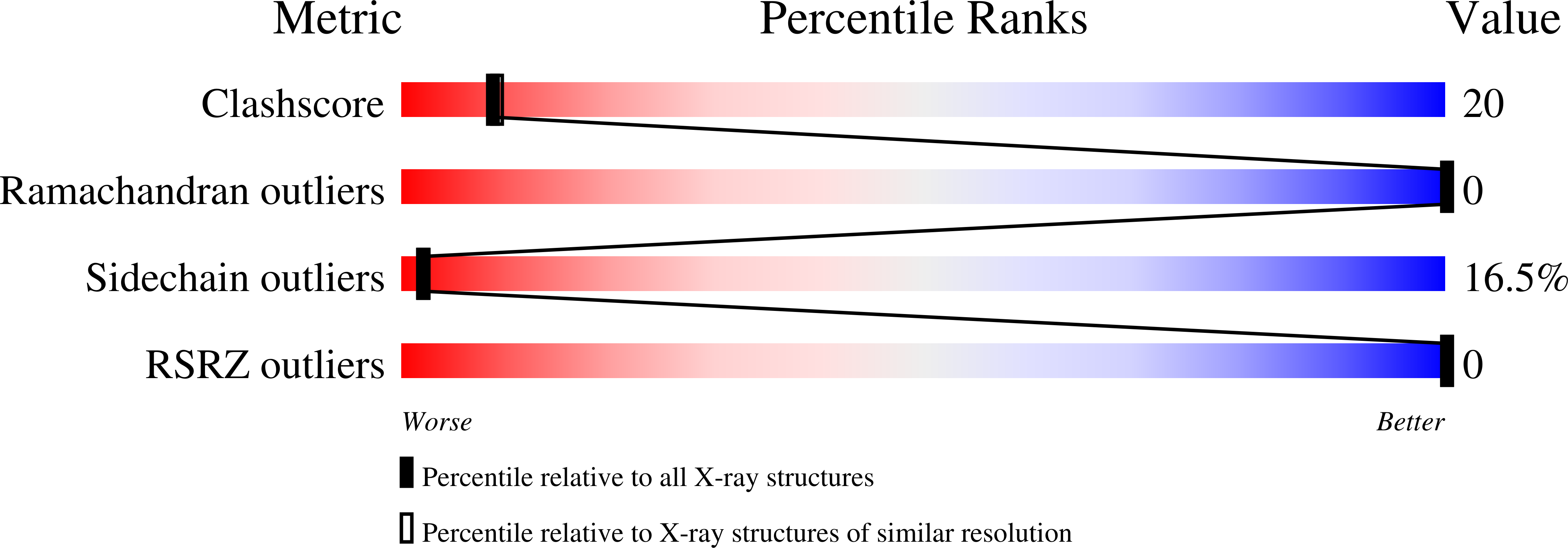Crystal structures of CheY from Thermotoga maritima do not support conventional explanations for the structural basis of enhanced thermostability.
Usher, K.C., de la Cruz, A.F., Dahlquist, F.W., Swanson, R.V., Simon, M.I., Remington, S.J.(1998) Protein Sci 7: 403-412
- PubMed: 9521117
- DOI: https://doi.org/10.1002/pro.5560070221
- Primary Citation of Related Structures:
1TMY, 2TMY, 3TMY, 4TMY - PubMed Abstract:
The crystal structure of CheY protein from Thermotoga maritima has been determined in four crystal forms with and without Mg++ bound, at up to 1.9 A resolution. Structural comparisons with CheY from Escherichia coli shows substantial similarity in their folds, with some concerted changes propagating away from the active site that suggest how phosphorylated CheY, a signal transduction protein in bacterial chemotaxis, is recognized by its targets. A highly conserved segment of the protein (the "y-turn loop," residues 55-61), previously suggested to be a rigid recognition determinant, is for the first time seen in two alternative conformations in the different crystal structures. Although CheY from Thermotoga has much higher thermal stability than its mesophilic counterparts, comparison of structural features previously proposed to enhance thermostability such as hydrogen bonds, ion pairs, compactness, and hydrophobic surface burial would not suggest it to be so.
Organizational Affiliation:
Institute of Molecular Biology and Department of Chemistry, University of Oregon, Eugene 97403, USA.