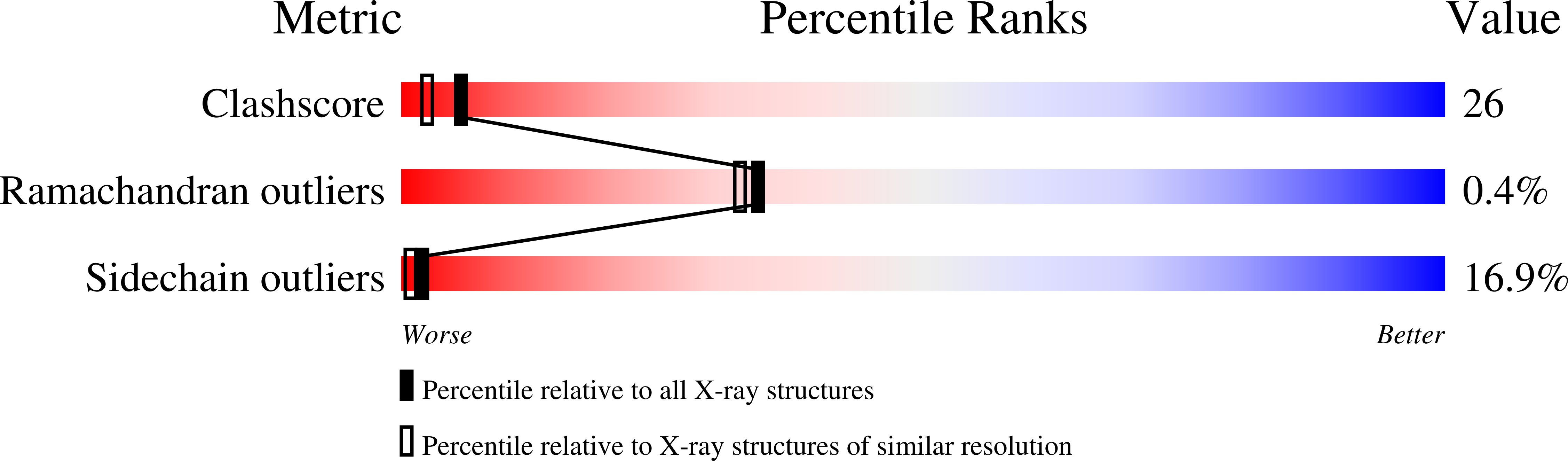Unexpected crucial role of residue 225 in serine proteases.
Guinto, E.R., Caccia, S., Rose, T., Futterer, K., Waksman, G., Di Cera, E.(1999) Proc Natl Acad Sci U S A 96: 1852-1857
- PubMed: 10051558
- DOI: https://doi.org/10.1073/pnas.96.5.1852
- Primary Citation of Related Structures:
1B7X, 1THP, 2THF - PubMed Abstract:
Residue 225 in serine proteases of the chymotrypsin family is Pro or Tyr in more than 95% of nearly 300 available sequences. Proteases with Y225 (like some blood coagulation and complement factors) are almost exclusively found in vertebrates, whereas proteases with P225 (like degradative enzymes) are present from bacteria to human. Saturation mutagenesis of Y225 in thrombin shows that residue 225 affects ligand recognition up to 60,000-fold. With the exception of Tyr and Phe, all residues are associated with comparable or greatly reduced catalytic activity relative to Pro. The crystal structures of three mutants that differ widely in catalytic activity (Y225F, Y225P, and Y225I) show that although residue 225 makes no contact with substrate, it drastically influences the shape of the water channel around the primary specificity site. The activity profiles obtained for thrombin also suggest that the conversion of Pro to Tyr or Phe documented in the vertebrates occurred through Ser and was driven by a significant gain (up to 50-fold) in catalytic activity. In fact, Ser and Phe are documented in 4% of serine proteases, which together with Pro and Tyr account for almost the entire distribution of residues at position 225. The unexpected crucial role of residue 225 in serine proteases explains the evolutionary selection of residues at this position and shows that the structural determinants of protease activity and specificity are more complex than currently believed. These findings have broad implications in the rational design of enzymes with enhanced catalytic properties.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, Box 8231, St. Louis, MO 63110, USA.