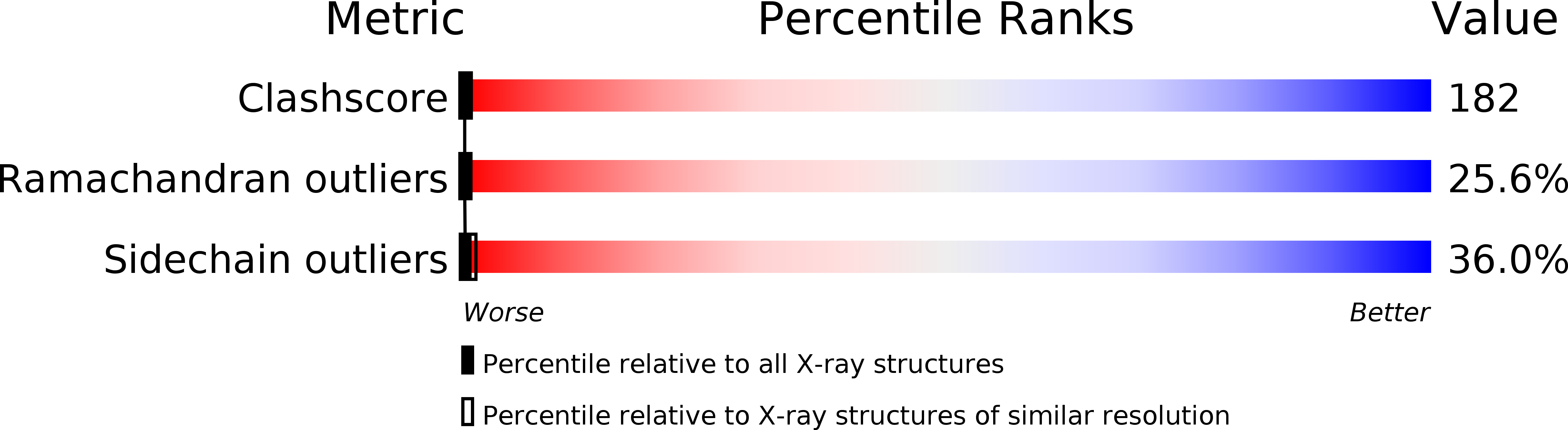Structure and possible catalytic residues of Taka-amylase A
Matsuura, Y., Kusunoki, M., Harada, W., Kakudo, M.(1984) J Biochem 95: 697-702
- PubMed: 6609921
- DOI: https://doi.org/10.1093/oxfordjournals.jbchem.a134659
- Primary Citation of Related Structures:
2TAA - PubMed Abstract:
A complete molecular model of Taka-amylase A consisting of 478 amino acid residues was built with the aid of amino acid sequence data. Some typical structural features of the molecule are described. A model fitting of an amylose chain in the catalytic site of the enzyme showed a possible productive binding mode between substrate and enzyme. On the basis of the difference Fourier analysis and the model fitting study, glutamic acid (Glu230) and aspartic acid (Asp297), which are located at the bottom of the cleft, were concluded to be the catalytic residues, serving as the general acid and base, respectively.