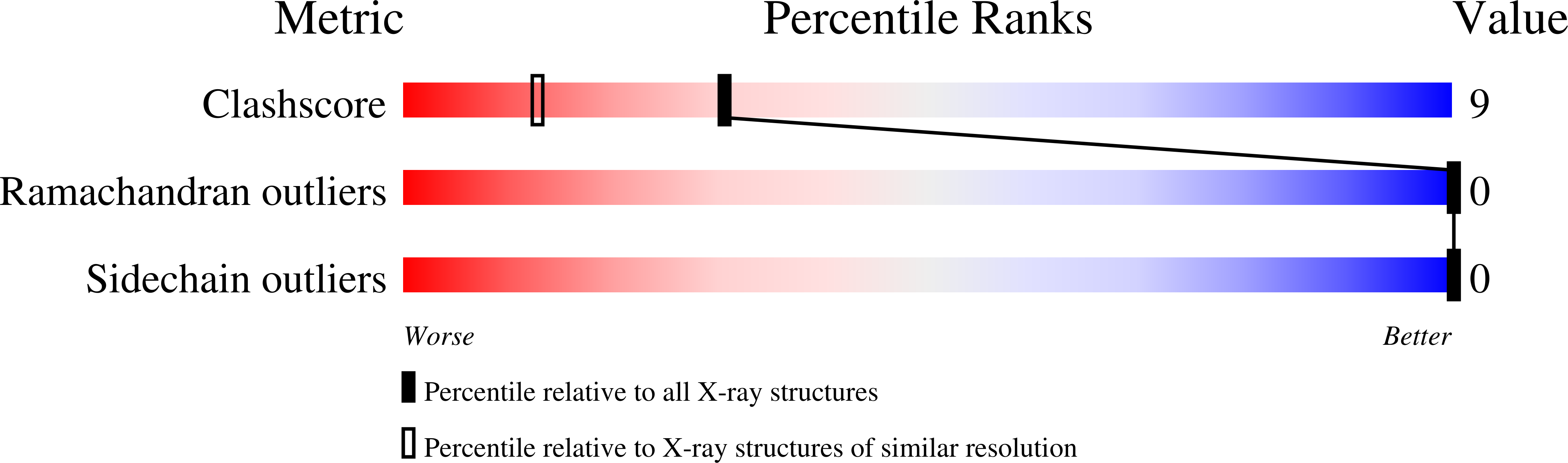
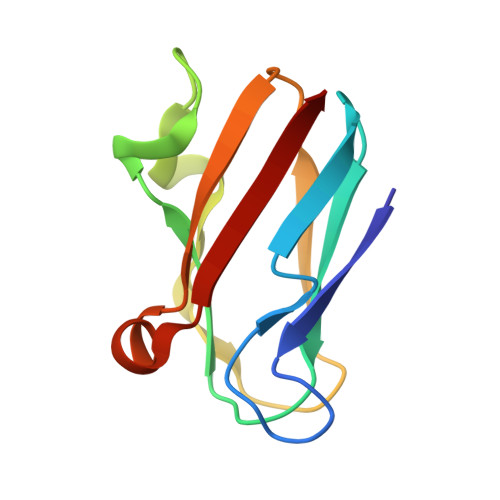
The 1.5-A crystal structure of plastocyanin from the green alga Chlamydomonas reinhardtii.
Redinbo, M.R., Cascio, D., Choukair, M.K., Rice, D., Merchant, S., Yeates, T.O.(1993) Biochemistry 32: 10560-10567
- PubMed: 8399201
- DOI: https://doi.org/10.1021/bi00091a005
- Primary Citation of Related Structures:
2PLT - PubMed Abstract:
The crystal structure of plastocyanin from the green alga Chlamydomonas reinhardtii has been determined at 1.5-A resolution with a crystallographic R factor of 16.8%. Plastocyanin is a small (98 amino acids), blue copper-binding protein that catalyzes the transfer of electrons in oxygenic photosynthesis from cytochrome f in the quinol oxidase complex to P700+ in photosystem I. Chlamydomonas reinhardtii plastocyanin is an eight-stranded, antiparallel beta-barrel with a single copper atom coordinated in quasitetrahedral geometry by two imidazole nitrogens (from His-37 and His-87), a cysteine sulfur (from Cys-84), and a methionine sulfur (from Met-92). The molecule contains a region of negative charge surrounding Tyr-83 (the putative distant site of electron transfer) and an exclusively hydrophobic region surrounding His-87; these regions are thought to be involved in the recognition of reaction partners for the purpose of directing electron transfer. Chlamydomonas reinhardtii plastocyanin is similar to the other plastocyanins of known structure, particularly the green algal plastocyanins from Enteromorpha prolifera and Scenedesmus obliquus. A potential "through-bond" path of electron transfer has been identified in the protein that involves the side chain of Tyr-83, the main-chain atoms between residues 83 and 84, the side chain of Cys-84, the copper atom, and the side chain of His-87.
Organizational Affiliation:
Department of Chemistry and Biochemistry and Molecular Biology Institute, University of California at Los Angeles, California 90024.