Sequence context induced antimicrobial activity: insight into lipopolysaccharide permeabilization.
Ghosh, A., Datta, A., Jana, J., Kar, R.K., Chatterjee, C., Chatterjee, S., Bhunia, A.(2014) Mol Biosyst 10: 1596-1612
- PubMed: 24714742
- DOI: https://doi.org/10.1039/c4mb00111g
- Primary Citation of Related Structures:
2MD1, 2MD2, 2MD3, 2MD4 - PubMed Abstract:
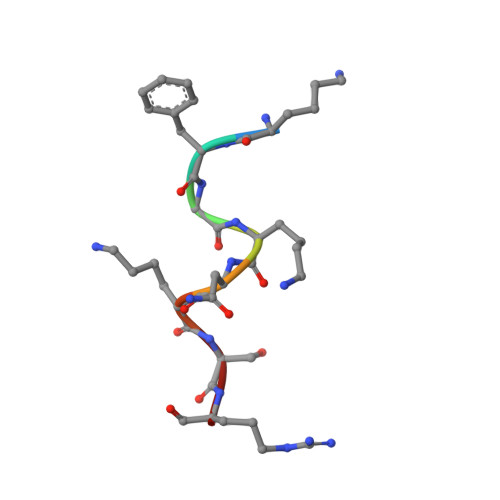
Lactoferrampin (WR17, Trp 268-Arg 284), an antimicrobial peptide, is known to have significant antibacterial and candidacidal activities. However, there are no previous studies explaining how WR17 permeabilizes the outer membrane of Gram negative bacteria and neutralizes endotoxins. In this study we used a series of assays like antimicrobial activity, calcein leakage, NPN dye uptake and endotoxin neutralization assay to show that the sequence context of WR17 modulates its multi-faceted activities. We determined the high resolution NMR structure of WR17 in LPS and found that the N-ter region forms a helix (Trp1-Phe11) and orients itself at an angle of 45° into the lipopolysaccharide (LPS) micelle, whereas the C-ter region (Lys13-Arg17) remains as a flexible extended random coil. We also verified this result through in silico molecular modeling simulation. Isothermal titration calorimetry showed that the interaction of WR17 and its analogues with LPS was primarily endothermic in nature. Using several fluorescence techniques such as anisotropy and red edge excitation shift assay we revealed motional restriction for Trp1 of WR17 in LPS. The distance between the indole ring of Trp1 of WR17 and the polar head group of LPS is around 7 Å, as obtained from the depth of insertion assay. Additionally, MD simulation demonstrated that the incorporation of the peptide in LPS is achieved with the help of the K(13)xK(15)xR(17) motif at the C-terminus. This novel anchoring "K(13)NKSR(17)" motif is currently being utilized in our ongoing research to design novel anti-endotoxic molecules.
Organizational Affiliation:
Biomolecular NMR and Drug Design Laboratory, Department of Biophysics, Bose Institute, P-1/12 CIT Scheme VII (M), Kolkata 700054, India. subhro_c@jcbose.ac.in bhunia@jcbose.ac.in.