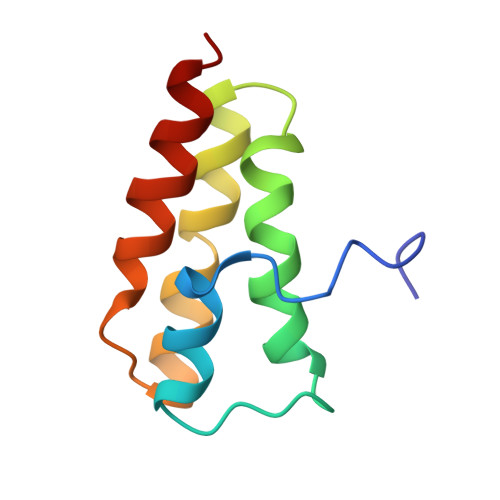
NMR Structure of the SARS-CoV Nonstructural Protein 7 in Solution at pH 6.5.
Johnson, M.A., Jaudzems, K., Wuthrich, K.(2010) J Mol Biol 402: 619-628
- PubMed: 20709084
- DOI: https://doi.org/10.1016/j.jmb.2010.07.043
- Primary Citation of Related Structures:
2KYS - PubMed Abstract:
The NMR structure of the severe acute respiratory syndrome coronavirus nonstructural protein (nsp) 7 in aqueous solution at pH 6.5 was determined and compared with the results of previous structure determinations of nsp7 in solution at pH 7.5 and in the crystals of a hexadecameric nsp7/nsp8 complex obtained from a solution at pH 7.5. All three structures contain four helices as the only regular secondary structures, but there are differences in the lengths and sequence locations of the four helices, as well as between the tertiary folds. The present study includes data on conformational equilibria and intramolecular rate processes in nsp7 in solution at pH 6.5, which provide further insights into the polymorphisms implicated by a comparison of the three presently available nsp7 structures.
Organizational Affiliation:
Department of Molecular Biology, The Scripps Research Institute, La Jolla, CA 92037, USA.