Solution structure of MAST2-PDZ complexed with the C-terminus of PTEN
Terrien, E., Wolff, N., Cordier, F., Simenel, C., Lafon, M., Prehaud, C., Delepierre, M.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Microtubule-associated serine/threonine-protein kinase 2 | 96 | Homo sapiens | Mutation(s): 0 EC: 2.7.11.1 | 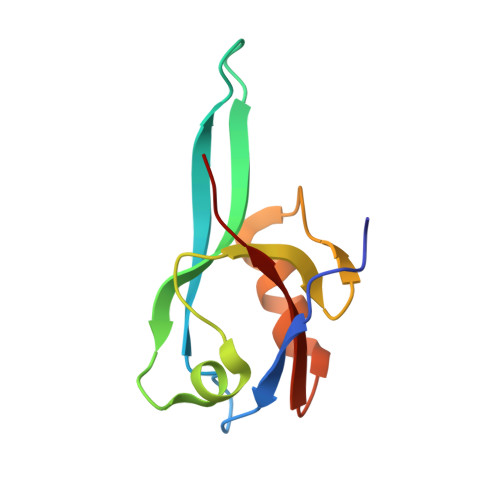 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q6P0Q8 (Homo sapiens) Explore Q6P0Q8 Go to UniProtKB: Q6P0Q8 | |||||
PHAROS: Q6P0Q8 GTEx: ENSG00000086015 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q6P0Q8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| C-terminus of Phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN | 13 | N/A | Mutation(s): 0 EC: 3.1.3.67 (UniProt), 3.1.3 (UniProt), 3.1.3.16 (UniProt), 3.1.3.48 (UniProt) | 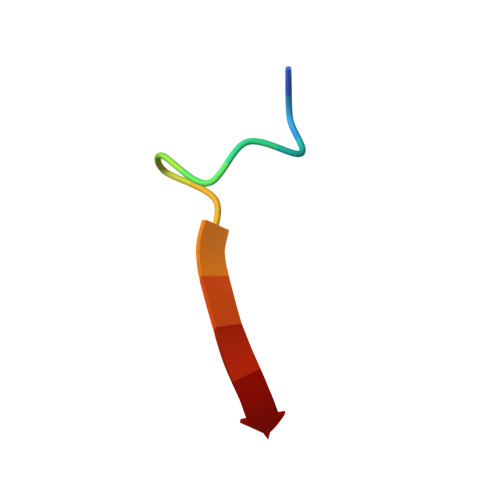 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P60484 (Homo sapiens) Explore P60484 Go to UniProtKB: P60484 | |||||
PHAROS: P60484 GTEx: ENSG00000171862 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P60484 | ||||
Sequence AnnotationsExpand | |||||
| |||||