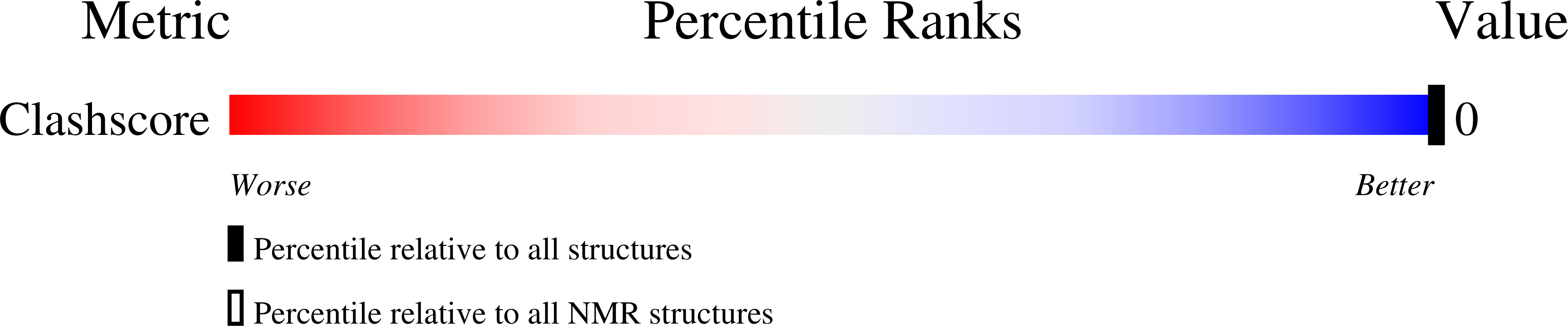Differential base stacking interactions induced by trimethylene interstrand DNA cross-links in the 5'-CpG-3' and 5'-GpC-3' sequence contexts.
Huang, H., Dooley, P.A., Harris, C.M., Harris, T.M., Stone, M.P.(2009) Chem Res Toxicol 22: 1810-1816
- PubMed: 19916525
- DOI: https://doi.org/10.1021/tx900225c
- Primary Citation of Related Structures:
2KNK, 2KNL - PubMed Abstract:
Synthetically derived trimethylene interstrand DNA cross-links have been used as surrogates for the native cross-links that arise from the 1,N(2)-deoxyguanosine adducts derived from alpha,beta-unsaturated aldehydes. The native enal-mediated cross-linking occurs in the 5'-CpG-3' sequence context but not in the 5'-GpC-3' sequence context. The ability of the native enal-derived 1,N(2)-dG adducts to induce interstrand DNA cross-links in the 5'-CpG-3' sequence as opposed to the 5'-GpC-3' sequence is attributed to the destabilization of the DNA duplex in the latter sequence context. Here, we report higher accuracy solution structures of the synthetically derived trimethylene cross-links, which are refined from NMR data with the AMBER force field. When the synthetic trimethylene cross-links are placed into either the 5'-CpG-3' or the 5'-GpC-3' sequence contexts, the DNA duplex maintains B-DNA geometry with structural perturbations confined to the cross-linked base pairs. Watson-Crick hydrogen bonding is conserved throughout the duplexes. Although different from canonical B-DNA stacking, the cross-linked and the neighbor base pairs stack in the 5'-CpG-3' sequence. In contrast, the stacking at the cross-linked base pairs in the 5'-GpC-3' sequence is greatly perturbed. The pi-stacking interactions between the cross-linked and the neighbor base pairs are reduced. This is consistent with remarkable chemical shift perturbations of the C(5) H5 and H6 nucleobase protons that shifted downfield by 0.4-0.5 ppm. In contrast, these chemical shift perturbations in the 5'-CpG-3' sequence are not remarkable, consistent with the stacked structure. The differential stacking of the base pairs at the cross-linking region probably explains the difference in stabilities of the trimethylene cross-links in the 5'-CpG-3' and 5'-GpC-3' sequence contexts and might, in turn, account for the sequence selectivity of the interstrand cross-link formation induced by the native enal-derived 1,N(2)-dG adducts.
Organizational Affiliation:
Department of Chemistry, Center in Molecular Toxicology, and Center for Structural Biology, Vanderbilt University, Nashville, Tennessee 37235, USA.