Functional Role of the Conserved Active Site Proline of Triosephosphate Isomerase.
Casteleijn, M.G., Alahuhta, M., Groebel, K., El-Sayed, I., Augustyns, K., Lambeir, A.M., Neubauer, P., Wierenga, R.K.(2006) Biochemistry 45: 15483
- PubMed: 17176070
- DOI: https://doi.org/10.1021/bi061683j
- Primary Citation of Related Structures:
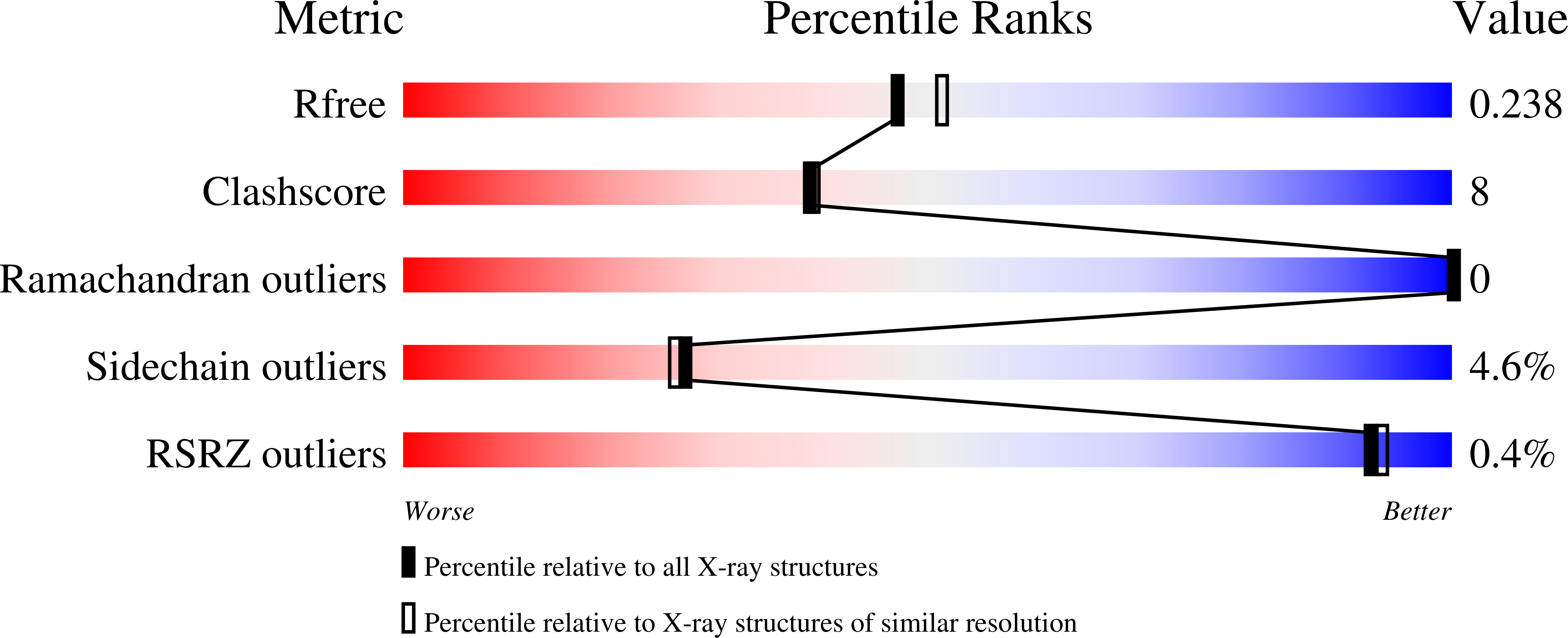
2J24, 2J27 - PubMed Abstract:
The importance of the fully conserved active site proline, Pro168, for the reaction mechanism of triosephosphate isomerase (TIM) has been investigated by studying the enzymatic and crystallographic properties of the P168A variant of trypanosomal TIM. In TIM, Pro168 follows the key catalytic residue Glu167, situated at the beginning of the flexible active site loop (loop 6). Turnover numbers of the P168A variant for its substrates are reduced approximately 50-fold, whereas the Km values are approximately 2 times lower. The affinity of the P168A variant for the transition state analogue 2-phosphoglycolate (2PG) is reduced 5-fold. The crystal structures of unliganded and liganded (2PG) P168A show that the phosphate moiety of 2PG is bound similarly as in wild-type TIM, whereas the interactions of the carboxylic acid moiety with the side chain of the catalytic Glu167 differ. The unique properties of the proline side chain at position 168 are required to transmit ligand binding to the conformational change of Glu167: the side chain of Glu167 flips from the inactive swung-out to the active swung-in conformation on ligand binding in wild-type TIM, whereas in the mutant this conformational change does not occur. Further structural comparisons show that in the wild-type enzyme the concerted movement of loop 6 and loop 7 from unliganded-open to liganded-closed appears to be facilitated by the interactions of the phosphate moiety with loop 7. Apparently, the rotation of 90 degrees of the Gly211-Gly212 peptide plane of loop 7 plays a key role in this concerted movement.
Organizational Affiliation:
Department of Process and Environmental Engineering and Bioprocess Engineering Laboratory, University of Oulu, P.O. Box 3000, FIN-90014 Oulu, Finland.