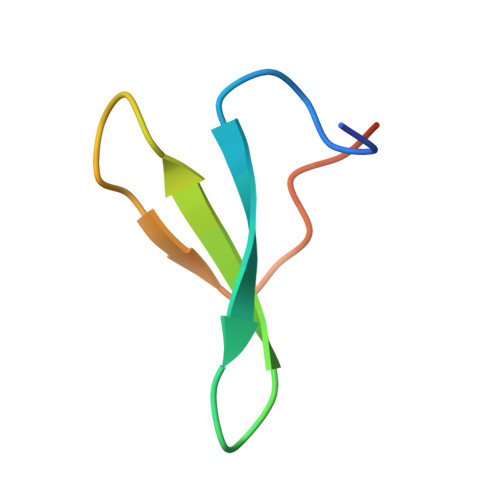
Structural Basis for Polyproline Recognition by the FE65 WW Domain.
Meiyappan, M., Birrane, G., Ladias, J.A.(2007) J Mol Biol 372: 970-980
- PubMed: 17686488
- DOI: https://doi.org/10.1016/j.jmb.2007.06.064
- Primary Citation of Related Structures:
2HO2, 2IDH, 2OEI - PubMed Abstract:
The neuronal protein FE65 functions in brain development and amyloid precursor protein (APP) signaling through its interaction with the mammalian enabled (Mena) protein and APP, respectively. The recognition of short polyproline sequences in Mena by the FE65 WW domain has a central role in axon guidance and neuronal positioning in the developing brain. We have determined the crystal structures of the human FE65 WW domain (residues 253-289) in the apo form and bound to the peptides PPPPPPLPP and PPPPPPPPPL, which correspond to human Mena residues 313-321 and 347-356, respectively. The FE65 WW domain contains two parallel ligand-binding grooves, XP (formed by residues Y269 and W280) and XP2 (formed by Y269 and W271). Both Mena peptides adopt a polyproline helical II conformation and bind to the WW domain in a forward (N-C) orientation through selection of the PPPPP motif by the XP and XP2 grooves. This mode of ligand recognition is strikingly similar to polyproline interaction with SH3 domains. Importantly, comparison of the FE65 WW structures in the apo and liganded forms shows that the XP2 groove is formed by an induced-fit mechanism that involves movements of the W271 and Y269 side-chains upon ligand binding. These structures elucidate the molecular determinants underlying polyproline ligand selection by the FE65 WW domain and provide a framework for the design of small molecules that would interfere with FE65 WW-ligand interaction and modulate neuronal development and APP signaling.
Organizational Affiliation:
Molecular Medicine Laboratory and Macromolecular Crystallography Unit, Division of Experimental Medicine, Harvard Institutes of Medicine, Harvard Medical School, Boston, MA 02115, USA.