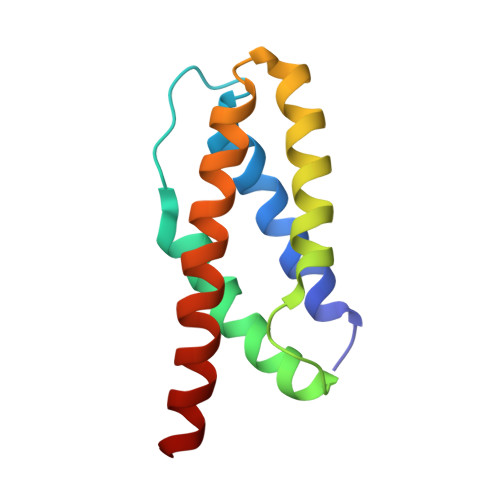
Structure and dynamics of a molten globular enzyme.
Pervushin, K., Vamvaca, K., Vogeli, B., Hilvert, D.(2007) Nat Struct Mol Biol 14: 1202-1206
- PubMed: 17994104
- DOI: https://doi.org/10.1038/nsmb1325
- Primary Citation of Related Structures:
2GTV - PubMed Abstract:
Although protein dynamics has been recognized as a potentially important contributor to enzyme catalysis, structural disorder is generally considered to reduce catalytic efficiency. This widely held assumption has recently been challenged by the finding that an engineered chorismate mutase combines high catalytic activity with the properties of a molten globule, a loosely packed and highly dynamic conformational ensemble. Taking advantage of the ordering observed upon ligand binding, we have now used NMR spectroscopy to characterize this enzyme in complex with a transition-state analog. The complex adopts a helix-bundle structure, as designed, but retains unprecedented flexibility on the millisecond timescale across its entire length. Moreover, pre-steady-state kinetics data show that binding occurs by an induced-fit mechanism on the same timescale as the enzymatic reaction, linking global conformational plasticity with efficient catalysis.
Organizational Affiliation:
Laboratory of Physical Chemistry, ETH Zurich, CH-8093 Zurich, Switzerland.