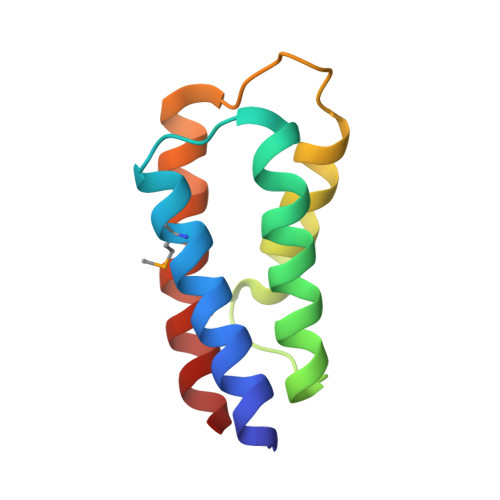
The crystal structure of the C-terminal domain of Vps28 reveals a conserved surface required for Vps20 recruitment.
Pineda-Molina, E., Belrhali, H., Piefer, A.J., Akula, I., Bates, P., Weissenhorn, W.(2006) Traffic 7: 1007-1016
- PubMed: 16749904
- DOI: https://doi.org/10.1111/j.1600-0854.2006.00440.x
- Primary Citation of Related Structures:
2G3K - PubMed Abstract:
The endosomal sorting complex I required for transport (ESCRT-I) is composed of the three subunits Vps23/Tsg101, Vps28 and Vps37. ESCRT-I is recruited to cellular membranes during multivesicular endosome biogenesis and by enveloped viruses such as HIV-1 to mediate budding from the cell. Here, we describe the crystal structure of a conserved C-terminal domain from Sacharomyces cerevisiae Vps28 (Vps28-CTD) at 3.05 A resolution which folds independently into a four-helical bundle structure. Co-expression experiments of Vps28-CTD, Vps23 and Vps37 suggest that Vps28-CTD does not directly participate in ESCRT-I assembly and may thus act as an adaptor module for downstream interaction partners. We show through mutagenesis studies that Vps28-CTD employs its strictly conserved surface in the interaction with the ESCRT-III factor Vps20. Furthermore, we present evidence that Vps28-CTD is sufficient to rescue an equine infectious anaemia virus (EIAV) Gag late domain deletion. Vps28-CTD mutations abolishing Vps20 interaction in vitro also prevent the rescue of the EIAV Gag late domain mutant consistent with a potential direct Vps28-ESCRT-III Vps20 recruitment. Therefore, the physiological relevant EIAV Gag-Alix interaction can be functionally replaced by a Gag-Vps28-CTD fusion. Because both Alix and Vps28-CTD can directly recruit ESCRT-III proteins, ESCRT-III assembly coupled to Vps4 action may therefore constitute the minimal budding machinery for EIAV release.
Organizational Affiliation:
European Molecular Biology Laboratory (EMBL), 6 rue Jules Horowitz, 38042 Grenoble, France.