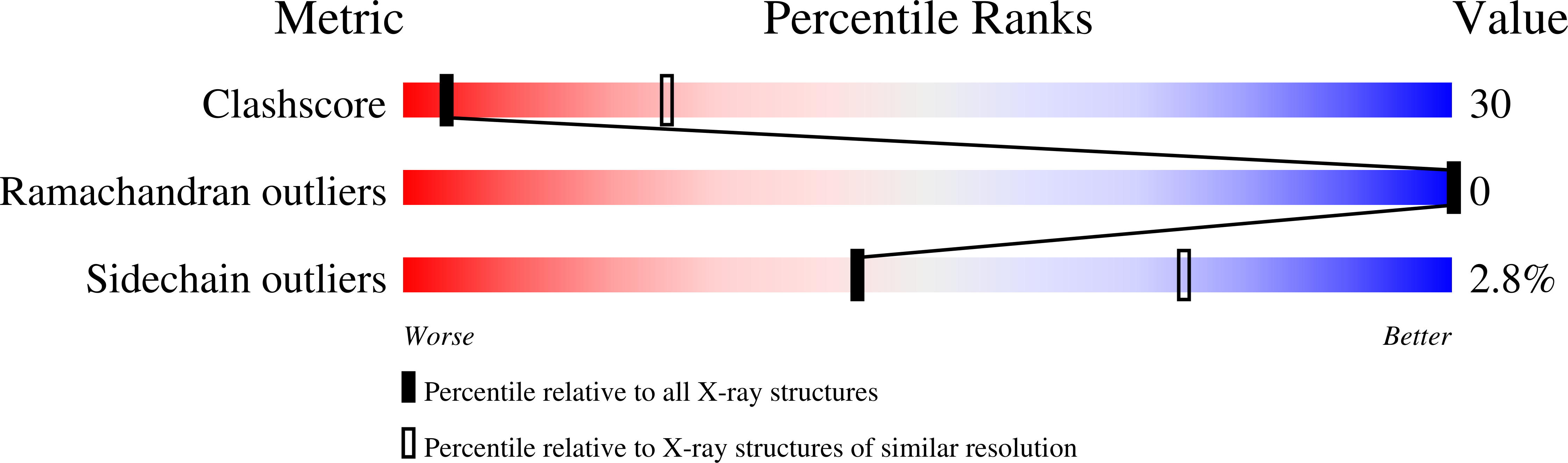Molecular Structure of Fd (F1, M13) Filamentous Bacteriophage Refined with Respect to X-Ray Fibre Diffraction and Solid-State NMR Data Supports Specific Models of Phage Assembly at the Bacterial Membrane.
Marvin, D.A., Welsh, L.C., Symmons, M.F., Scott, W.R.P., Straus, S.K.(2006) J Mol Biol 355: 294
- PubMed: 16300790
- DOI: https://doi.org/10.1016/j.jmb.2005.10.048
- Primary Citation of Related Structures:
2C0W, 2C0X - PubMed Abstract:
Filamentous bacteriophage (Inovirus) is a simple and well-characterized model system. The phage particle, or virion, is about 60 angstroms in diameter and several thousand angstrom units long. The virions are assembled at the bacterial membrane as they extrude out of the host without killing it, an example of specific transport of nucleoprotein assemblages across membranes. The Ff group (fd, f1 and M13) has been especially widely studied. Models of virion assembly have been proposed based on a molecular model of the fd virion derived by X-ray fibre diffraction. A somewhat different model of the fd virion using solid-state NMR data has been proposed, not consistent with these models of assembly nor with the X-ray diffraction data. Here we show that reinterpreted NMR data are also consistent with the model derived from X-ray fibre diffraction studies, and discuss models of virion assembly.
Organizational Affiliation:
Department of Biochemistry, University of Cambridge, Cambridge CB2 1GA, UK. d.a.marvin@bioc.cam.ac.uk