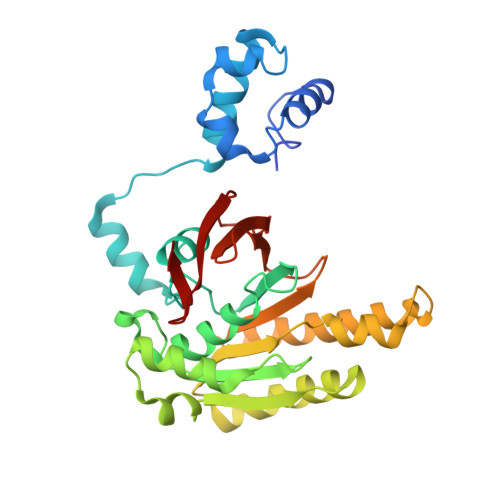
Three new structures of left-handed RADA helical filaments: structural flexibility of N-terminal domain is critical for recombinase activity
Chang, Y.W., Ko, T.P., Lee, C.D., Chang, Y.C., Lin, K.A., Chang, C.S., Wang, A.H.J., Wang, T.F.(2009) PLoS One 4: e4890-e4890
- PubMed: 19295907
- DOI: https://doi.org/10.1371/journal.pone.0004890
- Primary Citation of Related Structures:
2ZUB, 2ZUC, 2ZUD - PubMed Abstract:
RecA family proteins, including bacterial RecA, archaeal RadA, and eukaryotic Dmc1 and Rad51, mediate homologous recombination, a reaction essential for maintaining genome integrity. In the presence of ATP, these proteins bind a single-strand DNA to form a right-handed nucleoprotein filament, which catalyzes pairing and strand exchange with a homologous double-stranded DNA (dsDNA), by as-yet unknown mechanisms. We recently reported a structure of RadA left-handed helical filament, and here present three new structures of RadA left-handed helical filaments. Comparative structural analysis between different RadA/Rad51 helical filaments reveals that the N-terminal domain (NTD) of RadA/Rad51, implicated in dsDNA binding, is highly flexible. We identify a hinge region between NTD and polymerization motif as responsible for rigid body movement of NTD. Mutant analysis further confirms that structural flexibility of NTD is essential for RadA's recombinase activity. These results support our previous hypothesis that ATP-dependent axial rotation of RadA nucleoprotein helical filament promotes homologous recombination.
Organizational Affiliation:
Institute of Biochemical Science, National Taiwan University, Taipei, Taiwan.